As is true with virtually every aspect of the medical management of HIV infection, the care of HIV-infected women is an evolving process. Research continues to define the ways in which HIV-infected men and women are similar and different, findings that will continue to shape—and reshape—the standards of care that apply to both sexes.
| I. Epidemiology of HIV/AIDS Among Women | Top of page |
The number of women with HIV infection and AIDS has been increasing steadily worldwide. By the end of 2003, according to the World Health Organization (WHO), 19.2 million women were living with HIV/AIDS worldwide, accounting for approximately 50% of the 40 million adults living with HIV/AIDS.
“There are approximately 14,000 new HIV infections every day in the world,” Dr. Aberg noted. “More than 95% are in developing countries. Two thousand of these daily infections are in children under 15 years of age. The remaining 12,000 are in adults, of whom 50% are female and approximately 50% are 15 to 24 year2 old.”
In the United States, women comprise the fastest growing population of persons with AIDS. By the end of 2002, 159,271 adolescent and adult women in the United States were reported as having AIDS. The proportion of AIDS cases among women has more than tripled since the early years of the epidemic in the United States, from 7% in 1985 to 26% in 2002. Nonetheless, AIDS cases in adolescent and adult women have declined by 25% and have plateaued in the past five years, reflecting the success of antiretroviral therapies in slowing HIV disease progression.
Point estimates of cases diagnosed with AIDS by ethnicity reveal disproportionately high rates among African-American women (48.6 per 100,000), Hispanic women (11.3 per 100,000), American-Indian/Alaskan-Native women (5.8 per 100,000), Asian/Pacific Islander women (2.5 per 100,000), compared with white women (2.1 per 100,000). Furthermore, point estimates at the end of 2002 of persons living with HIV or AIDS in the 30 areas with confidential, names-based HIV reporting confirm a similar ethnic breakdown. As of the end of 2002, 48,948 cases are estimated in African-American women, 6,001 in Latina women, 269 in Asian/Pacific Islander women, 388 in American-Indian/Alaskan-Native women, and 16,000 in white women. In viewing these statistics, it is important to remember that African-American and Latina women represent 25% of all women in the United States, but comprise greater than 82% of all the AIDS cases among United States women.
The HIV prevalence and incidence of AIDS diagnoses annually among women over the age of 50 is also of concern. As explained by Dr. Aberg, adults over 50 years of age have long comprised 10% of all AIDS cases. However, by 1997, new cases rose to 11.6%; in 1998 the rate was 12.7%, and in 1999 the rate was 13.4%. While the increasing rates can be partly explained by the AIDS-delaying benefits of combination antiretroviral therapy—women infected earlier in life are now being diagnosed with AIDS later in life—it has been Dr. Aberg’s experience that many women are being infected later in life and overlooked in the process.
“Over the past three to four years, I have started seeing more and more women over the age of 50,” she explained. “Many of these women were married for 20 or so years, were recently divorced, and found themselves single again and having sex. For many of these women, pregnancy was no longer a concern and there really is a belief that women over a certain age simply aren’t at risk for STDs and HIV. But I’ve seen many older women in my clinic trying to come to terms with a new HIV diagnosis. What’s more, we’ve also seen elderly women admitted to the hospital with pneumonia, only to be misdiagnosed, because an HIV test was never performed. This is a population of women that continues to fall through the cracks.”
Mortality data among HIV-infected women in the United States have also been evaluated. In the CDC’s HIV Epidemiology Research Study (HERS), investigators evaluated HIV-associated and non-HIV-associated death rates and causes of death between 1993 and 1999 in HIV-infected and uninfected women (Smith, 2003). Causes of death were determined by review of death certificates and the National Death Index. In the 885 HIV-infected women and 425 HIV-negative women, 234 deaths and 8 deaths respectively occurred by December 31, 1999. Death rates, from any cause, were unchanged between the pre-combination antiretroviral therapy era (1993 to 1996) and the combination antiretroviral therapy era (1997 to 1999): 5.1 versus 5.4 deaths per 100 person-years. AIDS as a cause of death decreased from 58% of all deaths in 1996 to 19% in 1999, while combination antiretroviral therapy use increased to 42% by the end of 1999.
In spite of the relatively low death rate observed in the HIV-infected women, HIV-related mortality did decline over the course of the study, particularly in women with CD4+ counts less than 200 cells/mm3. Drug-related factors were prominent: for the 129 non-AIDS deaths, chronic hepatitis C virus (HCV) infection and injection drug use were strong predictors of mortality, but were not significant in the Cox model for 105 AIDS deaths. The regression analysis findings, along with the high percentage of non-AIDS deaths attributable to illicit drug use, suggest that high levels of drug use are a factor in this population that offset improvements in mortality from declining numbers of deaths due to AIDS.
Other study data have demonstrated marked decreases in population-based, all-cause mortality in women with HIV, driven by the large drops in HIV-related deaths. An example includes data from the Women’s Interagency HIV Study (WIHS) (Gange, 2002). Of the 2,059 HIV-positive women enrolled in WIHS, 692 reported AIDS-defining clinical conditions at their baseline visit (all women were enrolled between October 1994 and November 1995). Four-hundred ninety-two (71%) of the women with AIDS at baseline, and 1,199 (88%) of the women without AIDS at baseline, participated in at least one study visit after April 1, 1996.
During the course of follow-up, the incidence of AIDS showed a slight—although non-significant— increase between April 1995 and March 1996: 12.09 to 14.50 cases per 100 person-years. Between April 1998 and March 1999, however, there was a significant 14% semiannual decline over time to 5.07 cases per 100 person-years. When excluding the data prior to April 1996, the estimated semiannual decline was 23%.
The overall mortality rate also declined over the course of follow-up. The mortality rate among the women with AIDS at baseline was much higher than for those without AIDS at baseline. The mortality rate showed a strong, significant semiannual decline by 17% among those reporting AIDS at baseline between April 1995 and March 1999. In contrast, the mortality rate over the same time period among those women without AIDS at baseline did not show a statistically significant decline (-5% per six months). When excluding the data in the first year (April 1995 to March 1996), however, there was a statistically significant 11% semiannual decline in mortality between April 1996 and March 1999.
“The number of women in care, receiving treatment, is concerning,” Dr. Aberg added. While there are several potential reasons for this, the lack of social services available to women may be partly to blame. “If clinics had certain programs available, such as childcare, transportation, drug treatment, and safe housing, it’s likely that we’d improve access to care for women. I’ve seen programs such as these, work to the advantage of women in need of care.”
| II. Sex Differences: Transmission | Top of page |
Worldwide, more than 90% of all adolescent and adult HIV infections have resulted from heterosexual intercourse. Among AIDS cases in the United States reported in 2002, approximately 68% of HIV-positive women were infected via heterosexual intercourse; 29% were infected via injection drug use, and 3% were classified as other or not specified (see Figure 1). It is the high rate – and high risk – of HIV infection via sexual intercourse that has many public health officials concerned about the future of the HIV epidemic in women.
Figure 1. Proportion of AIDS Cases Among Adults and Adolescents, by Sex and Exposure Category Diagnosed in 2002, United States
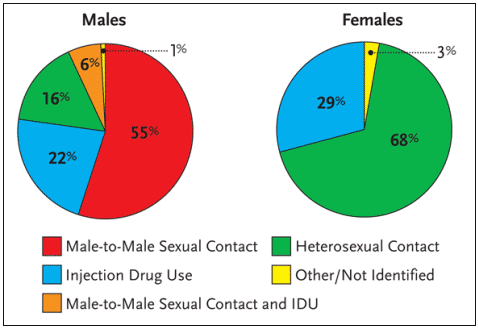
Research has documented that the cumulative incidence of HIV transmission from an HIV-infected woman to an HIV-negative man via unprotected sexual intercourse—that is, unprotected vaginal-penile sex over a sustained period of time with a fixed partner—is approximately 1% to 8%. Conversely, the cumulative incidence of HIV transmission from HIV-infected men to HIV-negative women via unprotected sexual intercourse is approximately 20%.
Reasons for the disparity have been outlined in the literature. First, semen tends to contain higher titers of HIV-RNA than vaginal secretions in HIV-positive individuals not receiving antiretroviral therapy. Second, viral entry via the mucosal tissues of the large vaginal and cervical surface area is much easier than entry via the urethra. Third, cervical ectopy—which has been shown to facilitate HIV infection—is common among young women. Finally, there are more HIV-infected men than there are women in the US.
| Disease Progression | Top of page |
Initial cohort studies conducted in the 1980s suggested that there were differences based on gender with respect to disease progression and survival. However, more recent studies indicate that gender does not necessarily predispose an HIV-infected individual to more rapid disease progression. These studies have confirmed that the most important predictors of survival are the AIDS-specific diagnosis, the CD4+ cell count, viral load, and age. “The early studies demonstrated that women were more likely to have limited access to care and were more likely to be diagnosed later in the course of their HIV infection,” Dr. Aberg said. “Female gender itself is not associated with more rapid disease progression.”
There is some evidence to suggest that women actually experience slower disease progression (Hubert, 2002). At the XIV International AIDS Conference, held in Barcelona in 2002, investigators with the French SEROCO cohort reported that women’s CD4+ cell counts were higher—and viral loads lower—for the first eight years after primary infection compared to men. More importantly, the relative risk (RR) for progression to AIDS was 0.34 for women compared to men, suggesting that the higher CD4+ cell count and lower viral load are protective. This difference was mitigated by adjustment for CD4+ cell counts and viral loads at baseline (RR=0.51) and by adjustment for time-dependent CD4+ cell counts and viral loads (RR=0.62). Only in this last comparison was the difference not significant, suggesting that the higher CD4+ cell counts and lower viral loads are mediating much of the difference.
As for opportunistic infection screening and prophylaxis, the recommendations are generally non-gender-specific. One opportunistic infection that does require careful consideration along gender lines is cytomegalovirus (CMV). In the United States, immunoglobulin G (IgG) antibodies to CMV are found in greater than 90% of men who have sex with men. In women, however, the prevalence of CMV antibodies is approximately 50%. “Clinicians have a tendency not to check CMV IgG,” Dr. Aberg explained. “Many claim that there’s no reason to check for CMV, given the high prevalence among men who have sex with men. However, the research shows that these statistics don’t apply to women and there are good reasons to know if someone is in fact negative for CMV IgG.” For example, CMV-negative patients in need of a transfusion should always receive CMV-negative blood to reduce the risk of CMV end-organ disease. Knowing a patient’s CMV serostatus can also be useful when dealing with a differential diagnosis.
Gender differences in viral load are also noteworthy. Several studies have demonstrated that women have lower viral loads than men at similar CD4+ cell counts. One such study, conducted by the CDC, involved a large cohort of 2,467 men and 1,309 women (Lee, 2001). Women with CD4+ counts between 0 and 199 cells/mm3 had viral loads that were 40% lower than men; women with CD4+ counts between 200 and 499 cells/mm3 had viral loads that were 48% lower; and women with CD4+ counts greater than 500 cells/mm3 had viral loads that were 57% lower. All of these results were statistically significant. “Clearly,” Dr. Aberg commented, “gender does play a role in viral load measurements prior to the initiation of antiretroviral therapy. However, the CDC has recommended that the prescribing guidelines not be changed for women. Multiple studies over the last decade highlight that women and men with equal access to care have similar rates of progression to AIDS and death despite the differences of viral set points. Gender simply is not a factor.”
| Response to Therapy: Efficacy and Side Effects | Top of page |
Antiretroviral therapy has been shown to be profoundly beneficial for the vast majority of HIV-infected patients. Encouragingly, data from some studies that succeeded in enrolling a large percentage of women indicate that there are no significant differences with respect to efficacy in women compared to men.
At the 15th International AIDS Conference, held in 2004 in Bangkok, Dr. Annemiek De Ruiter of St. Thomas’s Hospital in London and her colleagues presented the results of a subset analysis of Gilead Study 903, a clinical trial comparing tenofovir (Viread), lamivudine (Epivir), and efavirenz (Sustiva) to a combination of stavudine (Zerit), lamivudine, and efavirenz (De Ruiter, 2004). The study lasted three years and enrolled approximately 600 HIV-infected individuals, including 155 women (approximately 26% of the study population). All of the patients enrolled were naïve to antiretroviral therapy. Seventy-nine women were randomized to the tenofovir group and 76 were randomized to the stavudine group.
After 144 weeks of treatment, 67% of the women in the tenofovir group had HIV-RNA levels below 50 copies/mL, compared to 64% of women in the stavudine group. Among the men, 75% in the tenofovir group and 71% in the stavudine group had HIV-RNA levels below 50 copies/mL after three years. “Some people point out the trend suggestive of slightly lower virologic responses among women,” Dr. Aberg noted, “but the comparison between men and women was not statistically significant.”
Of interest, there was a statistically significant CD4+ cell count benefit—favoring women—after 144 weeks (see Figure 2). The mean CD4+ count increase among women receiving tenofovir was 314 cells/mm3, compared to 320 cells/mm3 among women receiving stavudine. As for the men, the mean CD4+ count increase was 246 cells/mm3 among those receiving tenofovir and 272 cells/mm3 among those receiving stavudine. For the comparison between the men and women with respect to CD4+ cell count gains, the p-value was less than 0.01.
Figure 2. Change in CD4 Cell Count (144 Weeks)Figure 2. Change in CD4 Cell Count (144 Weeks)
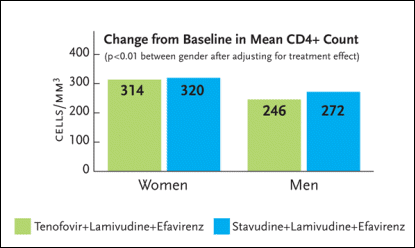
Gilead Study 903 was a three-year study enrolling 600 HIV-infected antiretroviral-naïve patients to receive either tenofovir (Viread), lamivudine (Epivir), and efavirenz (Sustiva) or stavudine (Zerit), lamivudine, and efavirenz. Seventy-nine women were randomized to the tenofovir group and 76 were randomized to the stavudine group. After 144 weeks of treatment, there was a statistically significant CD4+ cell count benefit favoring women. The mean CD4+ count increase among women receiving tenofovir was 314 cells/mm3, compared to 320 cells/mm3 among women receiving stavudine. As for the men, the mean CD4+ count increase was 246 cells/mm3 among those receiving tenofovir and 272 cells/mm3 among those receiving stavudine. For the comparison between the men and women with respect to CD4+ cell count gains, the p-value was less than 0.01.
Source:DeRuiter, 2004.
Dr. Aberg pointed out that there are some other notable treatment differences in women and men. For starters, women tend to weigh less and have a higher dose-per-kilogram ratio using standard antiretroviral doses than men. Women may also have changes in drug metabolism and clearance during various phases of their menstrual cycles. “Could these hormonal changes play a role in viral replication,” Dr. Aberg asked? “At this point in time, there are no firm answers.”
Unique drug-drug interactions are another important consideration. For example, the bioavailability of ethinyl estradiol—the active hormone in oral contraceptives—is significantly reduced when coadministered with ritonavir (Norvir), nelfinavir (Viracept), and nevirapine (Viramune). “In all of the clinical trials evaluating experimental antiretrovirals, we say that women must use two forms of birth control, given the concern surrounding oral contraceptives. Some women use the patch and some women use Depo-Provera, but the fact is we really don’t know how well these contraceptives mix with antiretrovirals. More work needs to be conducted in this area.”
The higher incidence of rash and hepatotoxicity in women using non-nucleoside reverse transcriptase inhibitors (NNRTIs) is also noteworthy. In a retrospective review of patients starting efavirenz or nevirapine during a three-year period, rash occurred in 14.6% of female patients, compared to 3% of male patients. This finding was statistically significant, enabling the authors to conclude that female sex is a strong independent risk factor for developing NNRTI- related rash (Mazhude, 2002). There are also hepatotoxicity data from a large cohort study involving 1,731 nevirapine-treated patients and 1,912 control patients (Imperiale, 2002). In this study, the relative risk of rash-associated hepatitis was 3.2 in women compared with men, and 9.8 in women with CD4+ counts greater than 250 cells/mm3 compared with those with lower CD4+ cell counts.
What about metabolic complications? Among non-HIV-infected adults, female sex is typically considered to be protective against dyslipidemia and atherosclerosis. Among HIV-infected women receiving antiretroviral therapy, there appear to be some important differences, although some of the data reported to date have yielded conflicting conclusions.
One study conducted by researchers at the University of Vienna Medical School in Vienna, Austria, set out to reveal potential sex differences in metabolic side effects of a newly initiated antiretroviral therapy regimen (Pernerstorfer-Schoen, 2001). The study enrolled 27 HIV-positive men and 13 HIV-positive women—all with HIV-RNA levels in excess of 10,000 copies/mL—along with 35 HIV-negative controls. Serum levels of lipids, insulin, and leptin were measured before beginning antiretroviral therapy, and at three and six months thereafter.
The antiretroviral regimen selected for the study—nelfinavir, didanosine (Videx), and stavudine—increased serum levels of triglycerides, leptin, and LDL cholesterol. Of note, these effects were more distinct in women. Fasting insulin levels and the LDL:HDL ratio increased only in the female HIV-infected patients, findings that were statistically significant. In contrast, endothelial activation—determined by monitoring levels of E-selectin—decreased more in men than in women.
Important gender-specific data are also available from ESS40002, a GlaxoSmithKline-sponsored study evaluating hyperlipidemia in antiretroviral-naïve patients taking zidovudine/lamivudine (Combivir) and abacavir (Ziagen), zidovudine/lamivudine and nelfinavir, or stavudine, lamivudine, and nelfinavir (Tashima, 2003). The study enrolled 254 adults, 50% of whom were women, at 34 sites in the United States, Panama, Dominican Republic, Guatemala, and Puerto Rico (see Figure 3).
Figure 3. ESS40002: Subgroup LDL Values--Change from Baseline to 96 Weeks
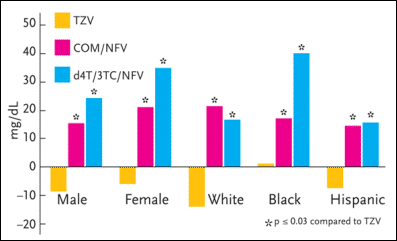
ESS40002 was a study evaluating hyperlipidemia in antiretroviral-naïve patients taking zidovudine/lamivudine (Combivir) and abacavir (Ziagen), zidovudine/lamivudine and nelfinavir (Viracept), or stavudine (Zerit), lamivudine (Epivir), and nelfinavir. The study enrolled 254 adults, 50% of whom were women. In the primary analysis of the entire study population, the mean LDL cholesterol and total cholesterol changes from baseline at week 96 significantly favored the zidovudine/lamivudine/abacavir group over the nelfinavir-based regimens. In a subset analysis, the female patients receiving either of the nelfinavir-based regimens had higher values for LDL cholesterol than men after 96 weeks.
Source:Tashima, 2003.
In the primary analysis of the entire study population, the mean LDL cholesterol and total cholesterol changes from baseline to week 96 significantly favored the zidovudine/lamivudine/abacavir group over the nelfinavir-based regimens. In a subset analysis, the female patients receiving either of the nelfinavir-based regimens had higher values for LDL cholesterol than men after 96 weeks. As for triglycerides, there was a trend toward greater increases in the male patients than in the female patients, although these data were not statistically significant.
In Gilead Study 903, changes in fasting lipid parameters after 144 weeks of therapy were analyzed in the women and men. Among women receiving stavudine/lamivudine/efavirenz, the mean total cholesterol increase was 58 mg/dL, the mean triglyceride increase was 71 mg/dL, and the mean LDL cholesterol increase was 34 mg/dL. Among men receiving this regimen, the mean total cholesterol increase was 58 mg/dL, the mean triglyceride increase was 151 mg/dL, and the mean LDL cholesterol increase was 24 mg/dL. In other words, as was documented in ES40002, the increase in triglyceride levels was less pronounced in women than in men.
Lipodystrophy-associated adipose tissue alterations among women—compared to those seen in men—have also been explored. In a study conducted at the University of Milan, Italy, adipose tissue changes were assessed in 2,258 HIV-infected patients (677 women) receiving treatment through one of six Italian clinical centers (Galli, 2003). In total, 750 (33%) patients reported a body-shape change consistent with either lipoatrophy or fat accumulation. Specifically, 29.5% of the men and 41.9% of the women reported at least one adipose tissue alteration. Women were significantly more likely to experience a combination of lipoatrophy and fat accumulation (22.4% vs. 9.7% respectively) or fat accumulation only ( 10.1% vs. 7.7% respectively) than men. Men (12.2%) were more likely than women (9.3%) to develop lipoatrophy. This last difference, however, was not statistically significant.
Over the past several years, reports have indicated that HIV-infected women have lower bone mineral density (BMD) than HIV-negative women. Some of these studies concluded that lower BMD was associated with HIV infection; others differed as to whether antiretroviral therapy ameliorates or exacerbates reduced BMD. In turn, Dr. Kathryn Anastos and her colleagues with the Women Interagency Health Study (WIHS) conducted an analysis to determine whether HIV-infected women have lower BMD than HIV-negative women and what role, if any, antiretroviral therapy plays in the development of this problem (Anastos, 2004).
The WIHS analysis involved a total of 232 women: 88 HIV-negative women and 184 HIV-positive women (94 of whom were receiving some form of antiretroviral therapy). Women taking exogeonous hormones, corticosteroids, or drugs used to treat osteoporosis were excluded. BMD measurements involved DEXA scanning at three sites: the spine, femoral trochanter, and femoral neck. Osteoporosis was defined as a t-score less -2.5; osteopenia was defined as a t-score between -1.0 and -2.5 (see Figure 4).
Figure 4. Osteopenia and Osteoporosis in W1HS
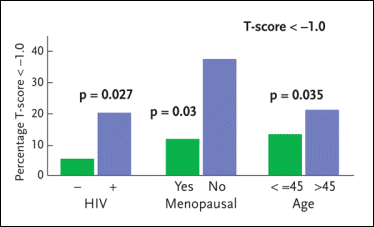
The Women Interagency Health Study (WIHS) has conducted an analysis to determine whether HIV-infected women have lower bone-mineral density (BMD) than HIV-negative women and to elucidate the role antiretroviral therapy plays in the development of this problem. The WIHS analysis involved a total of 232 women: 88 HIV-negative women and 184 HIV-positive women (94 of whom were receiving some form of antiretroviral therapy). Among the HIV-negative study volunteers, the prevalence of osteopenia/osteoporosis was 6.4%. Among the HIV-positive women not receiving antiretroviral therapy, the prevalence of osteopenia/osteoporosis was 18.9%; among the HIV-positive women receiving antiretroviral therapy, the prevalence of osteopenia/osteoporosis was 20.4%. Also reported here are rates of osteopenia/osteoporosis among menopausal and pre-menopausal women and women over 45 years of age compared to women 45 years of age or younger.
Source:Anastos, 2004.
HIV infection was associated with 6% to 8% lower BMD. However, the use of antiretroviral therapy did not significantly increase the risk of reduced BMD in the HIV-infected women. Among the HIV-negative study volunteers, the prevalence of osteopenia/osteoporosis was 6.4%. Among the HIV-positive women not receiving antiretroviral therapy, the prevalence of osteopenia/osteoporosis was 18.9%; among the HIV-positive women receiving antiretroviral therapy, the prevalence of osteopenia/osteoporosis was 20.4%. “While antiretroviral therapy did not prove to be a factor in the development of osteopenia or osteoporosis, it was interesting to see that women on nevirapine were more likely to have increased bone mineral density whereas women on long-term abacavir therapy were more likely to have decreased bone mineral density,” Dr. Aberg added. “The reason for this is not at all clear.”
Confusing matters are additional data from Gilead Study 903. BMD of the femoral head and spine was noted to decrease significantly from baseline in women who received tenofovir, compared to the women who received stavudine. This difference was not observed in the male patients.
| III. Gynecologic Care | Top of page |
There has been no shortage of data indicating that gynecologic complications can be more frequent, more severe, and less responsive to therapy among HIV-infected women when compared to their HIV-negative counterparts.
Since HIV and HPV are transmitted in somewhat similar fashions, the prevalence of HPV and cervical dysplasia among HIV-infected women would be expected to be relatively high. Data from the WIHS suggest that this is true.
Dr. Joel Palefsky of the University of California, San Francisco and his WIHS colleagues employed PCR to check for HPV in cervicovaginal lavage fluid collected from 2015 HIV-infected women and 577 HIV-negative controls (matched for age, drug use, and number of sexual partners) (Palefsky, 1999). Evidence of HPV infection was found in 58% of the HIV-positive women, compared with 26% of the controls.
HIV-positive women involved in this analysis were also more likely to be infected with multiple HPV types. Approximately 42% of the HIV-positive women, compared with 16% of the HIV-negative controls, had evidence of more than one type of HPV in lavage samples. Almost one-quarter of the HIV-positive women were infected with three or more types of HPV.
Also of interest was an association between HPV infection, CD4+ cell counts, and HIV-RNA levels in the HIV-positive women. As the CD4+ cell count declined, a greater percentage of HIV-positive women were found to have HPV: approximately 45% of women with CD4+ counts >500 cells/mm3, 55% of women with CD4+ counts between 200 and 500 cells/mm3, and 70% of women with CD4+ counts <200 cells/mm3 had PCR evidence of HPV infection. As for viral load, 71% of HIV-infected women with CD4+ counts above 500 cells/mm3 and plasma HIV-RNA levels in excess of 100,000 copies/mL were found to be positive for HPV, compared to 44% of HIV-positive women with similarly high CD4+ cell counts and less than 44,000 HIV-RNA copies/mL.
Additional data from WIHS involving Pap smears from 2,390 women—1,860 HIV-positive and 530 HIV-negative women—confirmed that abnormal cervical cytology is much more common among HIV-infected women compared to HIV-uninfected controls (Massad, 2001). Surprisingly, high-grade changes and cervical cancer were less common than expected over the 3.5-year time period of this study. Not surprisingly, the odds of progressing to a higher grade of abnormal Pap smear were associated with being both HIV- and HPV-positive and having a CD4 count below 200 cells/mm3 (Odds Ratio = 4.1 versus being HIV- and HPV-negative.)
Also from WIHS are data assessing the impact of antiretroviral therapy on cervical HPV infection in HIV-infected women (Minkoff, 2001). Women receiving antiretroviral therapy were 1.4 times more likely to demonstrate regression of their abnormal Pap smear. “How relevant is this to women living with HIV/AIDS,” Dr. Aberg asked? “Very. Abnormal Pap smears are extremely common among women with HIV and AIDS. If antiretroviral therapy can either halt progression of dysplasia, or improve regression, this would be extremely encouraging.”
Dr. Aberg also commented briefly on vaginal candidiasis. Research has established that vaginal colonization by Candida albicans is increased in HIV-infected women, injection drug users, and women on hypoglycemic agents. However, the actual clinical manifestation of vaginal candidiasis is equally as common in HIV-positive and HIV-negative women. “Vaginal candidiasis is not related to the CD4+ cell count or sexual contact,” Dr. Aberg said.
Oral colonization with C. albicans is also increased in HIV-positive women, tobacco users, and injection drug users. But unlike vaginal candidiasis, there is an increased risk of oral disease in HIV-positive women with low CD4+ cell counts, compared to HIV-negative women.
| Conclusion | Top of page |
In her concluding remarks, Dr. Aberg stressed that efforts are very much needed to expand prevention efforts and to increase availability of testing to at-risk women. “We still have too many women presenting late in the course of infection,” she said. “The research showing that women do not progress faster than men is important. Now we need to start making sure that women aren’t allowed to progress because of limited access to testing and services. Fear, isolation, and lack of information remain key issues to address.”
| References | Top of page |
