| Introduction | Top of page |
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) are two of the most prevalent persistent viral infections worldwide. Given their shared routes of transmission it is not surprising that HCV/HIV coinfection is relatively common. Globally, approximately 25% of patients with HIV are also infected with HCV (WHO, 2005). While the precise impact of HCV infection on the natural history of HIV infection is still controversial, uncontrolled HIV infection enhances the pathogenicity of HCV, leading to an increased morbidity and mortality in these patients. The introduction of highly active antiretroviral treatment (HAART) for HIV infection has led to dramatic reductions in morbidity and mortality due to AIDS (Palella, 1998). In these treated patients, HCV-related cirrhosis leading to hepatocellular failure and/or hepatocellular carcinoma (HCC) has become an increasingly important cause of morbidity and mortality, when expressed as a percentage of all deaths. It is not clear, however, whether there has been an absolute or only a proportional increase in morbidity following the introduction of HAART as rates of progression of liver disease in patients with well-controlled HIV infection receiving HAART are not substantively higher than in patients without HIV infection (Brau, 2006). Nonetheless, in the developed world, liver disease ranks behind only AIDS as the most common cause of death in HIV-infected patients (DAD Study, 2006).
Although HCV infection occurs commonly in HIV-infected patients overall, most patients infected with both HIV and HCV were infected with both viruses via injection drug use (IDU). In contrast, HIV-infected men-who-have-sex-with-men (MSM) who do not participate in IDU have essentially the same rate of HCV infection as the general population (Armstrong, 2006). In the last few years, however, there have been a number of reports of acute HCV infection in the HIV-infected MSM in urban centers in Europe (Browne, 2004; Gambotti, 2005; Gotz, 2005) and in the US (Luetkemeyer, 2006). An important finding from these studies is that in these populations of MSM, the major route of transmission appears to have been sexual, not parenteral. This article will review the literature relevant to acute HCV infection in HIV-infected MSM, with a focus on the research that has been performed regarding this recent epidemic.
| Definition of Acute HCV Infection | Top of page |
The definition of acute HCV infection is frequently misconstrued as suggesting medical severity of illness. Acute HCV infection, however, is defined simply as the initial 6-month period of HCV infection. HCV monoinfection is spontaneously cleared in approximately 25% of patients overall, and this clearance occurs in most cases during this initial 6-month period. Patients who do not have spontaneous clearance of their HCV infections (those who have persistent viremia) after 6 months are then defined as having chronic HCV infection. Acute HCV infection, far from being a symptomatic illness is, in fact, clinically apparent in only 20% of cases (Hoofnagle, 1997); combined with the fact that new HCV infections now occur predominately in people participating in IDU, who frequently do not engage in regular medical care, diagnosis of HCV infection during the acute phase is unusual.
The lack of a specific test for acute HCV infection further complicates the diagnosis of acute HCV infection. Therefore, operational definitions have been proposed with stringent criteria to attempt to minimize the misclassification of chronic but previously undetected HCV infection as acute infection. One such definition includes 1) documenting seroconversion to anti-HCV positivity; or 2) a serum alanine aminotransferase (ALT) level at least 10 times the upper limit of normal (ULN) (excluding other infective, metabolic, toxic and drug causes of hepatocellular damage) and detectable plasma levels of HCV RNA (Gerlach, 2003). Persistently seronegative chronic HCV infection has been reported in HIV-infected patients with low CD4 counts (George, 2002; Stapleton, 2004), and seroconversion to anti-HCV positivity may not have yet occurred upon presentation; absence of seroconversion to anti-HCV positivity, especially in patients with CD4 counts below 100 cells per �L should therefore not necessarily exclude the diagnosis of acute HCV infection in some patients.
| Epidemiology and Transmission | Top of page |
Transmission of HCV is parenteral (usually via IDU or transfusion), permucosal (sexual) or vertical. The epidemiology of an infection such as HCV is a function of its transmissibility and the population exposed to that route of infection. To understand the transmissibility of HCV, a comparison can be made with HIV. Examining the incidence of HCV infection following needle-stick injuries, HCV was approximately 10-times more transmissible than HIV (CDC, 2001). In contrast, HIV was 5- to 10-times more permucosally and vertically transmissible than HCV (Gibb, 2000; Verucchi, 2004). Heterosexual transmission of HCV infection is considered a very rare occurrence, and IDU remains the most common route of HCV transmission. A review by Mohsen and colleagues of a large UK HIV-infected cohort revealed that 9% were coinfected with HCV, and 66% of those infections were attributed to IDU (Mohsen, 2005). However, this pattern may be changing.
Beginning in 2004, a number of reports of acute HCV infection in HIV-infected MSM have emerged from large urban centers. In the first report, a retrospective review identified 25 cases in London between 1997 and 2002 (Browne, 2004). Although the amount of anti-HCV testing over this period remained stable, there was a significant increase in the proportion of positive tests, from 0.6% in 1997 to 9.3% in 2002 (P<.001). While this increase may have represented more appropriate testing and case ascertainment, it most likely indicated a true increase in acute HCV infection between those time periods. Interestingly, high-risk sexual practices, and not percutaneous exposures, were the main risk factors. Supporting the hypothesis of sexual transmission, 9 of the cases were also diagnosed with syphilis around the time of diagnosis of acute HCV infection. Since this initial report, more than 200 cases of acute HCV infection have been identified over the last 3 years in and around London. This epidemic has also been described by other groups in Europe (Gambotti, 2005; Gotz, 2005) and in the US (Luetkemeyer, 2006). In France, a cohort of 29 patients has been described from 3 centers (Gambotti, 2005). In the Netherlands, a cluster of 7 acute HCV cases was described in association with a surveillance study of rectal lymphogranuloma venereum (LGV), a recognized sexually transmitted disease (Gotz, 2005). It is noteworthy that all patients with acute HCV were HIV-infected MSM and that overall, patients from all countries reported permucosal rather than parenteral risk factors.
To elucidate the transmission of HCV in HIV-infected patients in London, we performed linked molecular (phylogenetic analyses) and clinical epidemiologic (questionnaire-based) studies (Danta, 2007). The cohort enrolled into the study consisted of 111 HIV-infected MSM with acute HCV infection recruited from 3 London clinics. The median age was 36 years and the HIV infection was well controlled (median CD4 count >500 cells/�L; 65% receiving HAART). A comparison made with the general HIV-infected population in the UK revealed this study group to be younger and have had a shorter duration of HIV infection.
Phylogenetic trees of the viral isolates (n=111) were constructed using the E1/E2 hypervariable region of the HCV genome to determine relatedness among the viral isolates. The finding of closely related HCV isolates among individuals would suggest common-source transmission. Phylogenetic analysis of the E1/E2 region revealed 7 monophyletic clusters of individuals, implying common-source transmission within these clusters of patients. Furthermore, multiple HCV genotypes were represented among these clusters, suggesting that this epidemic was not due to a specific more transmissible HCV strain, but rather to patient and/or environmental factors. Finally, a molecular clock analysis based on the rate of mutation within this region of the HCV genome revealed that 64% of HCV transmission events had occurred in the prior 10 years, dating to the introduction of effective HIV treatment (HAART).
Specific risk factors for HCV infection were explored in a case control study using a questionnaire instrument addressing multiple factors associated with HCV transmission, including detailed questions regarding sex practices, drug and alcohol use, and prior sexually transmitted infections (STIs) within the preceding 12 months, as well as attitudes toward HCV infection. This study enrolled 60 HIV-infected MSM with acute HCV (cases) and 130 HIV-infected MSM without HCV infection (controls). A key finding in this questionnaire study was that parenteral risk factors did not explain the vast majority of the HCV transmission; only 6.6% of controls versus 17.2% of cases (P=.08) reported IDU in the preceding 12 months. Therefore, 82.8% of the cases did not have IDU as a risk factor, which is the reversal of the more usual current pattern of HCV transmission (Alter, 2002). In our cohort, permucosal factors were substantially more important. For permucosal transmission of HCV to occur, however, there needs to be disruption of the mucosal barrier of the recipient, followed by exposure to infected body fluids. There were a number of possible mechanisms identified in this study which could disrupt the mucosa. Mucosal damage could arise through traumatic sexual practices or drug-sharing paraphernalia. In addition, STI leading to mucosal and/or skin lesions could potentiate HCV transmission. The disrupted mucosa then needs to be exposed to HCV-infected body fluids, a scenario that can occur in a number of ways. Unprotected intercourse could lead to exposure to semen or blood. For example, it has recently been demonstrated that HCV/HIV co-infection leads to increased rates and higher concentrations of HCV in the semen compared with HIV-negative men (Briat, 2005). Oral sexual practices could allow for exposure to saliva from which HCV has also been isolated (although HCV transmission has not been shown to occur via saliva). Furthermore, blood or secretions related to shared drug implements and sexual paraphernalia are other potential sources.
It has been recognized that a population of HIV-infected MSM exist who purposely seek HIV-positive partners with whom to have unprotected sex. This behavior, termed HIV-seroconcordant sexual partnering, has changed sexual behavior. A possible explanation for this behavior may be that unprotected intercourse between HIV-seroconcordant men is considered a �harm reduction� strategy. In the MSM community, some men intentionally seek unprotected intercourse with other HIV-infected men (termed �barebacking�) but these unprotected sexual practices put these men at increased risk of STIs, including HIV superinfection. Macdonald and colleagues at the Health Protection Agency in the UK found that the rates of HIV infection and other STIs, including syphilis, gonorrhea, genital herpes and chlamydia, have substantially increased among MSM (Macdonald, 2004). The rates in London were twice those of other parts of the UK. Interestingly, there has been a disproportionate rise in the diagnosis of syphilis in MSM, compared with heterosexual men in the UK, driven by large outbreaks in London and Manchester (Simms, 2005). Surveys of MSM performed over the last decade suggest that there is increasing high-risk behaviors including multiple partners, unprotected anal intercourse (UAI), and other mucosally traumatic sexual practices, such as fisting, rimming, use of sex toys, and sadomasochistic practices in this group (Nardone, 1997; Dodds, 2000). In a cross-sectional cohort study of MSM (N=8052) from venues in London between 1996 and 2000, increasing levels of UAI, especially in seroconcordant couples have been reported (Dodds, 2004). Another study, which reported the sexual practices of HIV-infected MSM over a 3-month period, found that if both partners were HIV-infected, UAI occurred in 91% as opposed to 34% of those who were HIV-negative or of unknown status (McConell, 2003). Among HIV-seroconcordant MSM factors predictive of high-risk behaviors were: lack of belief that STI and HIV superinfection were a problem, higher level of sexual compulsivity, and recreational drug use (Halkitis, 2005a). The same research group analyzed the practice of UAI (�barebacking�) and found an association with higher levels of sexual compulsivity, lower responsibility for safer sex and increased drug use�via both injection and noninjection (Halkitis, 2005b). Those identified as individuals engaging in UAI have more risk in terms of substance abuse, HIV transmission behaviors, and combining drug use with sexual risk taking.
Sexual partners sought via the Internet was an important, highly-associated risk factor in our study. This supports the increasing evidence that the Internet is an emerging risk environment for STIs (McFarlane, 2000). In a study of MSM in New York City, the Internet was determined, on multivariate analysis, to be an independent risk factor for transmission of syphilis (Wong, 2005). These findings may be understandable in that the Internet provides a convenient and confidential way of meeting sexual partners. Men who are HIV-infected may be able to disclose their status in a relatively safe environment, thereby establishing seroconcordance more easily. While it is controversial whether the Internet leads to an overall increase in high-risk behavior, there is good evidence that in HIV-infected MSM it does increase risky behavior (Bolding, 2005; Hospers, 2005). Therefore, the Internet probably represents an epiphenomenon associated with HCV transmission by a third direct transmission factor, such as high-risk sex.
FIGURE 1. Model of Variables Interaction and HVC Transmission in HIV-Positive MSM Describing the Complex Contribution of Factors Within the Sexual and Drug Milieu.
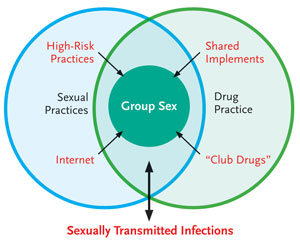
Our questionnaire-based, case-control study revealed distinct differences between the cases and controls with respect to their individual sexual practices. A larger proportion of cases participated in high-risk sexual practices with more partners than controls. The unsafe sexual practices of UAI, rimming, fisting and sharing of sexual paraphernalia were all significantly associated (P<.001) with acute HCV infection. Theoretically, such practices could be associated with permucosal transmission of HCV infection as a result of mucosal trauma and exposure to infected secretions. It should be stated that the controls also had a relatively high level of risk, with more than one third participating in UAI.
Group sex is a situation where these sexual factors could potentially amplify HCV transmission. Group sex practices were more common in the cases than controls. Of those participating in group sex, higher-risk behaviors were more common in the cases than in the controls with regard to UAI and fisting. Individuals are exposed to high-risk practices with multiple partners, increasing the risk of transmission. It is conceivable that multiple transmissions could occur in these situations, but there is a paucity of data on group sex behavior. Clatt and colleagues performed qualitative research among HIV-positive MSM participating in group sex parties in New York. They found that individuals used parties as a means of serosorting with very high rates of unprotected intercourse and previous drug use (Clatts, 2005). Our study revealed little evidence of safe sex practices such as condom or glove use.
It is unsurprising that there were more STIs in the cases compared with the controls. There are 2 interpretations of this phenomenon. Most likely, this is a reflection of the high-risk sexual behavior of the cases. As in our cohort, higher-risk sexual behavior has been associated with the increased risk of STIs. Key to the transmission of STIs is the number of sexual partners. While both cases and controls had a high number of median sexual partners, the cases had significantly more (30 versus 10, P<.001). Analysis of the UK NATSAL study revealed that numbers and types of sexual partners were the dominant individual and population risk factors for the acquisition of STIs (Fenton, 2005). The study reported a disproportionate burden of STIs in highly sexually active MSM living in Greater London, with almost 50% of the STI occurring in fewer than 10% of the people who reported more than 10 sexual partners. It is possible that STIs may also directly contribute to HCV transmission through percutaneous and/or mucosal lesions and/or the presence of infected inflammatory cells. The relationship between STIs and HIV transmission has been recognized for some time (Stamm, 1988).
Cases were more likely to have participated in sex under the influence of recreational drugs than controls. �Club drugs� were more commonly used by the cases, including ketamine, methamphetamines, gamma hydroxybutyrate (GHB), poppers, LSD and ecstasy. Many of these drugs are intranasally taken, and this route of drug taking was shared considerably more often by the cases than controls. In addition, the cases used intra-anal drugs more frequently than controls. There are 2 possible mechanisms whereby drug use may increase HCV transmission: sharing of the instrument used to take the drugs, and higher-risk sexual behaviors resulting from the drug use. Interestingly, both intranasal and rectal drug use were more frequently seen in the cases than controls, and although it is theoretically possible that infected implements led to transmission of HCV, intranasal transmission of HCV is rare (CDC, 1998). Regarding rectal transmission, it is likely that the effect of the drugs caused disinhibition and heightened sexual arousal, leading to the mucosal trauma associated with HCV transmission.
In summary, our study identified a number of potential factors associated with the recent transmission of HCV infection (Danta, 2007). These transmission factors form a complex interaction between sexual and drug risk behaviors. While significant in univariate analysis, many of these factors are highly correlated and have been difficult to disentangle in the multivariate analysis. The final multivariate model identified group sex as the only independent factor associated with acute HCV infection in the study cohort. While, among the identified risk factors, the association with acute HCV infection was the strongest for group sex, this factor in itself is not a specific mechanism for transmission of HCV. It is more likely to have represented a mechanism whereby a third factor within the milieu of sexual and drug factors was responsible for the HCV transmission. This study suggests that the mechanism(s) may be either mucosally traumatic sexual practices or the sharing of drug implements, both of which could biologically transmit the infection. However, to define these specific factors, a detailed qualitative study at the event level would be required. Figure 1 graphically represents the study cohort. A number of factors in the univariate analysis appear to be contributing to the recent transmission. First, mucosally traumatic sexual practices including UAI, rimming, fisting, use of sex toys and sadomasochistic practices could all theoretically transmit HCV infection. Internet use may contribute to this by facilitating the access of HIV-positive individuals to these high-risk practices. �Club drugs� may increase participation in high-risk sexual activities. The sharing of drug implements, particularly intranasally and intra-anally, may also directly lead to HCV infection. Finally, the role of STIs is unclear (represented by a 2-way arrow). They most likely represent the result of the high-risk sexual practices. However, STIs may also be potentiating HCV transmission through mucosal and/or skin lesions. Using the factors identified in this study, high-risk individuals such as those participating in group sex could be identified. These factors need to be the focus of any public health intervention to prevent the spread of HCV in HIV-infected MSM.
| Immunology and Natural History | Top of page |
The natural history of HCV infection depends upon the host-viral interaction. This has been predominantly studied in HCV monoinfection. A successful immune response to HCV infection requires strong, broad and sustained HCV-specific CD4 and CD8 T-cell responses (Diepolder, 1995; Gerlach, 1999). The CD4 T-cells are vital for priming and sustaining CD8 cytotoxic T-cells, which lead to control of the infection. Gerlach and colleagues demonstrated that patients who failed to clear HCV infection either did not mount CD4 T-cell responses or, after initial virologic control, did not sustain these responses with a consequent relapse of HCV viremia (Gerlach, 1999). This finding was reinforced by Thimme and colleagues who analysed CD4 T-cell responses in healthcare workers exposed to HCV (Thimme, 2001). Whilst 2 patients initially developed strong CD4 T-cell responses, they developed persistent infection following loss of these responses. These studies were performed on peripheral blood samples; intrahepatic CD4 T-cell responses in the acute phase in humans have not been studied because liver biopsy is not usually performed early in the course of HCV infection. However, in chimpanzee studies, intra-hepatic CD4 T-cell responses were identified only in those animals with controlled HCV (Thimme, 2002). Hepatitis C virus-specific T-cell responses have been identified against a wide range of epitopes, particularly the nonstructural proteins (Diepolder, 1997; Lamonaca, 1999). These HCV-specific T-cell responses are persistent and have been detectable up to 2 decades following resolution of HCV in a group of women who had been infected via human Rhesus immunoglobulin (Takaki, 2000).
The appearance of CD8 T-cells is temporally associated with control of HCV viremia. Tetramer studies have shown that CD8 T-cells can take several weeks to expand after the onset of HCV infection (Shoukry, 2003). Analysis of individuals following needlestick exposure to HCV found that CD8 T-cells at week 7 were associated with substantial elevations of serum ALT, but that they did not produce interferon-gamma (IFN-?). Later, the CD8 T-cells recovered their ability to produce IFN- ?, which was associated with a considerable drop in viremia and resolution of liver disease (Thimme, 2001). Similar to CD4 T-cell responses, CD8 T-cell epitopes have been identified to all HCV proteins. In those patients spontaneously clearing HCV infection, these responses have also been shown to be vigorous and multispecific (Gruner, 2000; Lechner, 2000). Studies have confirmed that CD8 T-cell responses are necessarily sustained for long-term clearance of HCV (Takaki, 2000; Thimme, 2002).
Preexisting HIV infection substantially impairs the cell-mediated responses to HCV antigens (Harcourt, 2006). During chronic HCV-infection, cell-mediated responses are difficult to detect and often narrow in focus. A comparison of acute HCV infection in HIV-infected patients from 1 center (N=55; Royal Free Hospital, London) was made with a cohort of 8 Italian HCV monoinfected individuals (Danta, 2006). The vast majority (95%) of HIV-HCV coinfected patients in the London study did not clear their HCV infection during the acute phase and progressed to chronic HCV infection. This proportion is notably higher than that for the Italian monoinfected controls (62%) and reported historic controls (75%), suggesting that concurrent HIV infection favors HCV persistence. Furthermore, the median HCV viral load in the acute phase was higher by approximately 0.5 to 1 log IU/mL than both the Italian and German historic monoinfected controls (Gerlach, 2003), implying poorer virologic control of HCV in HIV coinfection during the acute phase of HCV infection (Figure 2). Other small studies of acute HCV infection in HIV-infected patients have reported spontaneous clearance rates of 0 to 24% (Gambotti, 2005; Gilleece, 2005).
Comparison of IFN-? ELISpot CD4 T-cell responses between 14 HIV-infected patients and 8 HIV-uninfected patients with acute HCV infection revealed that while CD4 T-cell responses were present to some HCV antigens in the early phase of HCV infection in HIV-infected patients, these responses lacked the breadth and magnitude that is probably necessary for control of HCV infection. This contributed to the higher rate of viral persistence and higher HCV viral loads in this cohort compared with HIV-uninfected patients (Danta, 2006). The responses of the HIV-infected individuals were most clearly defective against the HCV NS3, NS4, and NS5 proteins, which have been shown to be particularly important for the clearance of HCV infection (Diepolder, 1995). Comparison between HIV-infected hemophiliac patients with acute or chronic HCV infection revealed that a higher proportion of patients with acute HCV infection had measurable CD4 T-cell IFN-? ELISpot responses, but typically only against core peptides (35% vs 7%) (Harcourt, 2006). Failure to develop a vigorous, multispecific and persistent cell-mediated response in HCV infection is postulated to occur as a result of primary T-cell failure and/or exhaustion, T-cell dysfunction, viral escape mutation, or a combination of these (Neumann-Haefelin, 2005). HIV infection is likely to influence these mechanisms through alterations in CD4 T-cell survival, antigen-presenting cell function and/or disruption of lymphoid architecture.
The replication of HCV is rapid and error prone and has been shown to play an important role in the evasion of host immune responses (Bukh, 1995). The complexity of the HCV population (the number of viral variants) and its diversity (mean pairwise genetic distance of clones) during HCV infection of HIV-infected patients has only been studied during chronic HCV infection and the data are conflicting with evidence for both increased and decreased diversity (Sherman, 1996; Toyoda, 1997; Mao, 2001; Roque Afonso, 2002). We therefore studied evolution of HCV in the acute phase of infection by analyzing longitudinal sequencing of the E1/E2 region of the HCV genome in 8 HIV-infected patients. The complexity and diversity at multiple time points were studied along with the degree of immune pressure, described by the ratio of change in nucleotides of the sequence leading to amino acid changes (dN/dS ratios). A dN/dS ratio of greater than 1 suggests a degree of positive immune selection pressure on that sequence. Analysis of the viral evolution revealed an increase in HCV diversity and complexity over time in 5 out of 8 patients. However, for the same region all patients had dN/dS ratios that were below 1. This suggests that the HCV evolution observed was not driven by immune selection pressure but more likely was the result of viral mutation and random genetic drift. This is entirely consistent with the lack of cell-mediated immune responses observed (Danta, 2006).
We believe it is somewhat problematic to compare our studies of acute HCV in HIV-infected patients with those published on HIV-uninfected patients, as our patients tended to be clinically asymptomatic (having been diagnosed through routine lab screening during regular HIV care), and the cohorts of HIV-uninfected patients with acute HCV infection were mostly symptomatic. The spontaneous clearance rate of HCV in HIV-uninfected patients has been estimated in a recent meta-analysis to be 25% (Micallef, 2006). In published studies of HIV-infected patients, this rate may be lower, with rates reported from 0 to 24% (Table 1). Our study of 55 patients shows a clearly lower rate of spontaneous clearance rate of only 5%, with only 3 patients who had spontaneous clearance of HCV infection all having been jaundiced. It has been reported that female sex, of which there were none in our study, and symptomatic disease, which was rare in our cohort, favors spontaneous clearance, which may explain the disparate results compared to studies of HIV-uninfected patients.
| Management | Top of page |
No consensus yet exists for the management of acute HCV infection in HIV-infected patients, but a strong case can be made for early intervention with combination therapy since acute HCV infection has previously been shown in HIV-uninfected patients to be very sensitive to treatment. Recombinant interferon-alpha (IFN-?)? monotherapy has yielded sustained virologic responses (SVR, defined as HCV RNA negativity 6 months post HCV treatment) of 98% (Jaeckel, 2001), that have been maintained in long-term follow-up (Wiegand, 2004). Similar results have been reproduced in trials using pegylated IFN-???, a longer acting interferon (Kamal, 2004; ?Nomura, 2004; Santantonio, 2005). Since 20% to 50% of individuals will spontaneously clear HCV infection (Lauer, 2001), in HIV-uninfected patients, it has been suggested that delay of treatment for up to 6 months from the onset of HCV infection might not appear to impact treatment outcome while allowing the possibility of spontaneous eradication of HCV in some patients (Gerlach, 2003). Treatment of chronic HCV infection in HIV-infected patients has a relatively poor outcome compared to treatment of chronic HCV infection in HIV-uninfected patients with SVRs between 27% and 40% with 12 months of therapy (Carrat, 2004; Chung, 2004; Torriani, 2004). There are emerging data from observational studies suggesting that treatment of acute HCV infection in HIV-infected patients leads to better outcomes than treatment during chronic HCV infection in these patients (Table 1). Various IFN-based regimens, with or without ribavirin, resulted in SVR between 0 and 91% (Danta, 2005; Gilleece, 2005; Vogel, 2005; Luetkemeyer, 2006; Serpaggi, 2006). These studies suggest that combination pegylated IFN and weight-based ribavirin during acute HCV infection appears to result in higher SVR than during chronic HCV infection in HIV-infected patients. Successful eradication of HCV infection abolishes the complications of chronic coinfection. Therefore, the acute phase of HCV infection provides an ideal period in which to intervene to prevent the progression to chronic HCV infection. Based on this early literature, we believe that the combination of pegylated IFN -? and weight-based ribavirin to be the regimen of choice in the treatment of acute HCV infection in HIV-infected patients.
| Table 1. Clinical Summary and Treatment of Described Cohorts of Acute HCV in HIV-Positive Individuals. | ||||||||||
| Cohort | Number | Median Age (y) |
Jaundiced (%) | Genotype 1 and 4 (%) | AntiHCV Positive (%) | Spontaneous Eradication(%) | Number Treated | Type of Treatment | Length of Treatment (weeks) | SVR (%) |
| Gambotti et al, 2004 | 29 | 40 | 3 (10) | 21 (72) | 29 (100) | 0 (0) | NA |
NA | NA | NA |
| Serapaggi et al, 2006 | 12 | 40 | 2 (17) | 11 (92) | 10 (100) | 0 (0) | 10 | ifn-α2/peg ifn-α2 + Ribavirin | 24 | 0 (0) |
| Gilleece et al, 2005 | 50 | 37 | 3 (6) | 43 (86) | 50 (100) | 12 (24) | 27 | peg ifn-α2b + Ribavirin | 24 | 16 (59) |
| Danta et al, 2005 | 55 | 36 | 6 (11) | 47 (85) | 52 (95) | 3 (5) | 24 | peg ifn-α2a/b + Ribavirin | 24-48 | 17 (71) |
| Vogel et al, 2005 | 11 | 36 | 5 (45) | 10 (91) | 11 (100) | 0 (0) | 11 | ifn-α2/ peg ifn-α2 +/- Ribavirin | 24 | 10 (91) |
| Gotz et al, 2005 | 7 | NA | 0 (0) | 7 (100) | 7 (100) | 0 (0) | NA | NA | NA | NA |
| Luetkemeyer et al, 2005 | 9 | 45 | 5 (56) | 6 (67) | 9 (100) | 2 (22) | 4 | peg ifn-α2a + Ribavirin | 24-48 | 2 (50) |
| * hcv indicates hepatitis C virus; hiv, human immunodeficiency virus; svr, sustained virologic response; na, not available; ifn, interferon; peg, pegylated. | ||||||||||
The treatment of acute HCV in HIV-positive individuals requires a multidisciplinary approach. Healthcare workers should attempt to identify the transmission risk factor(s), which could provide a focus for prevention of reinfection, increase awareness about ongoing risks, and identify other individuals at risk. If the patient is on HAART, the physician needs to review the medication regimen since important interactions have been identified between HAART and combination HCV treatment. Ribavirin enhances the intracellular phosphorylation of didanosine (ddI), which increases the risk of mitochondrial toxicity. This combination has been associated with life-threatening lactic acidosis and multiorgan dysfunction (Lafeuillade, 2001). Based on the use of the data from RIBAVIC study, there is now an FDA recommendation against the combination of ribavirin and ddI. The British HIV Association (BHIVA) recommends avoiding the combination of zidovudine (AZT) or ddI with ribavirin therapy (Nelson, 2005). The clinical pathway used at the Royal Free Hospital, London, is outlined in Figure 3. In this clinical pathway, we wait 12 weeks to allow spontaneous eradication of the HCV before instituting therapy. However, in our experience spontaneous clearance is rare and usually occurs only in patients who present with icterus.
| Conclusion | Top of page |
Acute HCV infection substantially differs between HIV-infected and HIV-uninfected patients in its epidemiology, natural history, immunology, and virology, and is becoming an increasingly major problem among HIV-infected MSM.
We have found multiple HCV variants among HIV-infected MSM with acute HCV infection in the London area, implying that any intervention needs to be of an appropriately broad scope to encompass the at-risk population. Importantly, permucosal rather than percutaneous transmission is occurring as evidenced by the findings of our case-control study. Factors identified among cases included high numbers of sexual partners, participation in group sex (mucosally traumatic sexual practices), use of illicit drugs (�club drugs�), sharing of drug-taking implements, and use of the Internet to meet other men. These factors are strikingly different from the usual epidemiology of acute HCV infection, where the majority of HCV is transmitted parenterally via IDU.
Based on some of these observations, a number of recommendations can be made for identifying, controlling, and treating acute HCV infection in MSM. There needs to be increased surveillance for HCV both to identify cases and assess the scope of the problem. In response to the marked increase in cases in London, routine screening for HCV infection has been introduced in some London clinics. Through the identification of specific transmission factors, higher-risk individuals can be identified for increased surveillance and education-based interventions. All the cases identified are HIV-infected men, suggesting factors specific to this particular group are important in the HCV transmission. Specific education-based strategies could then be focused on this group with the aim of decreasing exposure to sexually traumatic practices and drug use and increasing safe sex practices. Finally, healthcare workers also need to be educated about the implications of this emerging problem.
In our study, HCV persistence was the overwhelming outcome of acute HCV infection of HIV-infected patients, with rates of progression to chronicity considerably higher than those for HIV-uninfected patients. Pre-existing HIV infection appears to impact the adaptive host responses during acute HCV infection. Treatment algorithms therefore need to ensure that infected individuals are treated in a timely fashion with appropriate antiviral regimens. Such algorithms will maximize the chance of viral clearance during the acute phase. We believe that treatment during the acute phase is warranted.
Finally, this HCV epidemic in HIV-infected MSM has resulted from a noteworthy change in patient behavioral risk factors, reverting to the high-risk behavior in some that was prominent during the 1970s and 1980s which resulted in the initial spread of HIV infection among MSM. To mitigate this important and ongoing epidemic, these behaviors need to be the focus of a concerted prevention effort on the part of public health specialist, clinicians and HIV-infected patients themselves.
| Acknowledgements | Top of page |
The research discussed relevant to the London cohort was a collaboration between the Royal Free Hospital, Chelsea; Westminster Hospital, Brighton and Sussex General Hospital; Oxford University; and the Royal Free and University College Medical Schools (RF&UCMs), London. This work was supported by Special Trustees of RF&UCMs Fellowship and a Peter Samuel Fellowship. Specifically, Professor Geoff Dusheiko, Professor Caroline Sabin, Dr Nasser Semmo and Dr Paul Klenerman should be thanked.
| References | Top of page |
