| Human Papillomavirus Background | Top of page |
With more than 100 known types, the human papillomavirus (HPV) is the most common sexually transmitted infection with approximately 75% of sexually active adults acquiring one or more genital HPV types at some point in their lifetimes.1 HPV is spread from skin to skin because HPV lives only in keratinocytes. When used 100% of the time, condoms have been shown to reduce HPV transmission by up to 70%.2 Although HPV infects a high proportion of sexually active men and women, most do not show clinical manifestations of the infection.
HPV types 6, 11, 42, 43, and 44 are the causative agents of anogenital condylomas and cervical flat condylomas, which are not considered to be cancer precursors. HPV types 16 and 18 are found in a high proportion of advanced cervical intraepithelial neoplasia (CIN) and are considered to be precursors to invasive cancer in about 50% and 20% of all cervical cancer cases in the United States, respectively. The percentages of cervical cancers associated with HPV types 31, 33, and 35 are lower and vary from country to country.3 This review will discuss the pathogenesis of HPV in HIV-positive patients, provide an update regarding anal intraepithelial neoplasia (AIN), and outline screening guidelines for patients at risk for HPV-related disease.
| Natural History of Cervical HPV Infection | Top of page |
The progression of HPV-related cervical disease is well characterized and proceeds through distinct phases. The Bethesda System Classification, which categorizes cervical cytology, organizes HPV-associated lesions into low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL) and invasive cancer (Figure 1).4,5
Figure 1. From Condyloma to Cancer
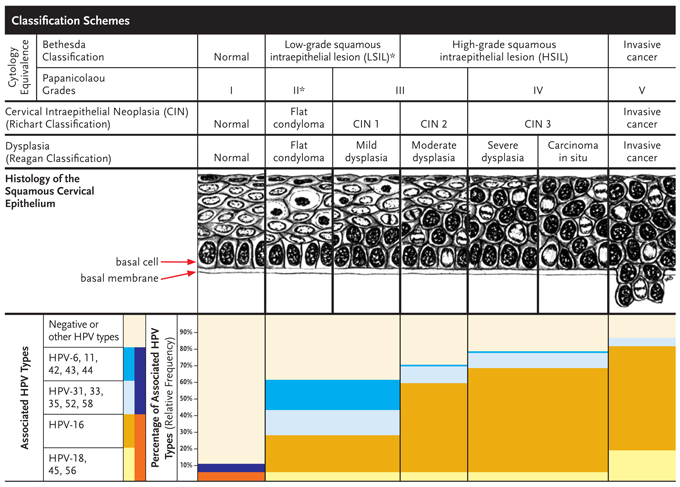
*LSIL also includes atypical squamous cells of unknown significance (ASCUS).
Adapted with permission from William Bonnez, MD and ASM Press, Washington DC.5
LSIL corresponds to the histologic diagnoses of flat condylomas and CIN 1, whereas HSIL corresponds to the histologic diagnoses of CIN 2 and 3. As the degree of severity increases, the more oncogenic HPV types become increasingly predominant.4 In most cases, the time from development of initial HPV infection to CIN 2-3 is believed to be less than five years, whereas the progression of CIN 2-3 to invasive cancer may take several decades.6
| Anal HPV Coinfection and AIN in HIV-negative and -positive Women | Top of page |
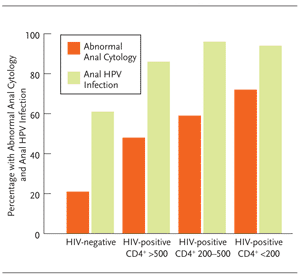
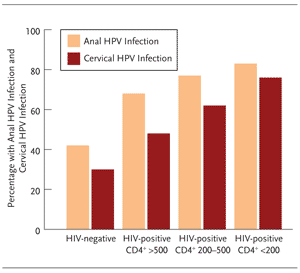
In women, the prevalence of anal HPV is actually higher than the prevalence of cervical HPV, independent of HIV status and level of immunosuppression (Figure 8) and the incidence of anal cancer is 7-fold higher in HIV-positive women compared with HIV-negative women at high risk for HIV.16,29,31 The relationship between anal HPV infection and cervical HPV infection is poorly understood, as is the effect of anal HPV infection on the biology of cervical disease.
| Anal Cytology Screening Recommendations for HIV-positive Men and Women | Top of page |
The New York State Public Health AIDS Institute created recommendations for the care of all HIV-infected individuals that include screening for AIN.42 The recommendations for anogenital examination include:
- Inquire about anal symptoms at baseline and annually; symptoms include itching, bleeding, diarrhea, and pain;
- Perform visual inspection of the anal region at baseline and annually;
- Perform a digital rectal examination (DRE) at baseline and annually;
- Perform anal cytology at baseline and annually in:
- Patients with abnormal anal Pap smear findings should be referred for high resolution anoscopy (HRA) and/or examination with biopsy.42
a. Men who have sex with men (MSM)
b. Patients with history of anogenital condylomas
c. Women with abnormal cervical/vulvar histology; and
The benefits of anal screening may be realized at all stages of anal disease.43 With proactive screening in at-risk populations, smaller lesions are more likely to be detected. Smaller lesions tend to be easier to treat than larger lesions, and can be treated in the office with simple techniques such as cryotherapy with liquid nitrogen or topical application of trichloroacetic acid.36 In most cases, larger or multifocal lesions may also be treated in the office using newer modalities such as infrared coagulation.44,45 Larger lesions can be treated surgically.46 While chemoradiation therapy is the standard of care for anal cancer it should not be used to treat AIN.47 At later stages of disease, when the lesions are too large or widespread to be removed, there is still benefit to screening because it allows for careful monitoring for progression to invasive cancer.
As with cervical cancer, survival from anal cancer is improved by early diagnosis.48 Anal cancer screening techniques, such as the DRE to feel for masses, combined with visualization through HRA and biopsy, are important in the care of patients with AIN. Performing the DRE to feel for subcutaneous masses is particularly important to fully assess for presence of anal cancer, because these tumors may be entirely below the epithelial surface and can occasionally be missed on visual inspection of the surface using HRA. DRE should be performed at least annually on all patients at risk for anal cancer, and is an especially important tool in clinical settings where anal cytology or HRA are not available. At present there are no data on the effect of AIN screening on the incidence of anal cancer. Future studies are needed to examine this relationship.
| Future Approaches and Vaccination | Top of page |
While the efficacy of therapeutic vaccines to treat AIN is still under investigation, much attention has been given to the recently approved preventive HPV vaccines. The vaccines work by expressing a major capsid protein encoded by the L1 gene of HPV in eukaryotic cells.49 The L1 proteins, or virus-like particles (VLP) auto-assemble into a three-dimensional structure that closely resembles that of the native HPV viral capsid. When injected into humans, these VLPs stimulate immunity against the real HPV virus.
There are two different vaccines, one of which is currently FDA-approved in the United States. Merck has created Gardasil, which is an FDA-approved quadrivalent vaccine comprised of four HPV types, including 16 and 18 (oncogenic types) and 6 and 11 (wart-producing types).49 GlaxoSmithKline has created Cervarix, which is a bivalent vaccine composed of HPV types 16 and 18; this vaccine is pending FDA approval.50
Among women who had no evidence of prior exposure to HPV types in the vaccine, vaccination is nearly 100% effective to prevent disease associated with those HPV types in the vaccine.49,50 However, the vaccines generally do not have any effect on development of CIN if a woman has already been infected with the HPV types in the vaccine.49 The vaccines would therefore not be expected to be as efficacious in women with extensive sexual exposure. There is some confusion about the reported efficacy of the vaccines since the efficacy will depend on the proportion of women in the study population (or general population) that has not previously been exposed to the HPV types in the vaccine. Another source of reduced vaccine efficacy is disease due to HPV types other than 16, 18, 6 or 11. In published studies of Gardasil, the efficacy was only 39% since it included patients with prior exposure to HPV vaccine types, as well as development of CIN in some patients from HPV types not included in the vaccine.49,51 Since the vaccine is recommended for girls 9 to 12 years of age, it is expected that the efficacy will more closely resemble that of patients without prior exposure, ie, nearly 100% protection against disease associated with vaccine types, and relatively little protection against disease due to non-vaccine types.
These vaccines have the potential to prevent both penile and anal HPV infection and their associated diseases in men. Studies of both heterosexual men and MSM are in progress, and if the vaccines are shown to be effective in this setting, discussions will be initiated to determine the merits of vaccinating both men and women. Since AIN can be challenging to treat, and since a high proportion of anal cancers are associated with HPV 16, vaccines may be an excellent prevention strategy for anal cancer in the long-term, provided that at-risk individuals receive the vaccine before they have been exposed to HPV. In the case of MSM and the psychosocial dynamics of coming out and seeking health care, this may be especially challenging if boys in the general population are not routinely vaccinated prior to sexual debut.
HPV vaccination presents another challenge in the HIV-positive population. HIV-positive men and women are clearly at high risk of disease due to HPV types in the vaccines, and may benefit from vaccination.52 However, it is not known how effective vaccination will be in the HIV-positive population since immunosuppression may attenuate development of protective titers of HPV antibodies. The safety of the vaccine has not yet been studied in HIV-positive adults and this population may already have been exposed to some or all of the types in the vaccine. Overall, HIV positive patients most likely will have a reduced efficacy of vaccination, and as with MSM in general, the optimal strategy to prevent HPV infection in this population would be to vaccinate all boys and girls before sexual debut. Given the current debate about vaccinating girls prior to sexual debut, such a recommendation would undoubtedly stimulate even more vigorous debate.
| Conclusions | Top of page |
The incidence of AIN and anal cancer is much higher in HIV-positive women and MSM than in the general population, and HAART has had little or no impact on this trend. There is a growing need for definitive guidelines to assess for AIN, and with better treatment options available, it is even more crucial to identify these patients at an earlier stage. New York is the first state to institute guidelines for anal cytology screening in HIV-positive patients. If anal cytology and HRA are not available, all high-risk patients should be screened with a DRE as there are many benefits to early detection of anal cancer. HPV vaccines have the potential to reduce the incidence of anal cancer, but more studies are needed to evaluate the efficacy in patients infected with HIV.
| Peer Review Contributions | Top of page |
We thank Stephen E. Goldstone, MD, Charles John Gonzalez, MD, Jeff W. Huyett, APRN, and Michael N. Pierce, MD for their critical review of this manuscript.
| References | Top of page |

Comments
Commenting Guidelines
Editor Comment
Posted by Marnie G. Henderson on 10/16 at 06:34 PM
Thank you for your interest in commenting on “Anogenital Human Papillomavirus Coinfection and Associated Neoplasia in HIV-positive Men and Women” by Drs. Jason Bratcher and Joel Palefsky. We welcome your thoughtful discussion concerning this article. Please review the above commenting guidelines.
We look forward to your comments.
James F. Braun, DO
Editor-in-Chief, The PRN Notebook
Reader Comment
Posted by galmayer on 10/20 at 01:15 PM
Thank you Drs. Palefsky and Bratcher for this excellent review of the relationship of HPV infection to anogenital cancers in HIV-infected men and women. New York State’s AIDS Institute has taken the bold and appropriate first step of incorporating anal cancer prevention and screening into the guidelines, but it’s clear that we don’t have adequate numbers of trained anoscopists to screen all the HIV-infected men and women in the state. Future efforts around this issue should address the disparity between the demand for screening and the resources. Training more providers to do the screening and prevention is essential, but further research may also address this issue. Specifically, one important research question is at what age should screening begin. Do sexually-active adolescents need anal Pap smears? Or is it safe to wait until say… age 30 because of the biological differences between anal cancer and cervical cancer? More clearly defining the at-risk population would help us focus our resources on those most at need.
Reader Comment
Posted by wbonnez on 10/20 at 02:41 PM
In their excellent review on screening for HPV disease in patients with HIV, Jason Bratcher and Joel Palefsky point out that the New York State Department of Health (NYSDOH) recommended in September 2007 the use of anal cytology for the prevention of anal cancer, the first state or federal agency to do this. Men who have sex with men, any patient with a history of anogenital condylomas, and women with abnormal cervical and/or vulvar histology are to have a baseline anal cytology then repeated annually.
The concept of anal cytology screening is modeled after cervical cytology screening for the prevention of cervical cancer. The same HPV genotypes, mostly 16 and 18, are found in both cervical and anal cancers, and the oncogenic potential of these viruses has been well established over the past 30 years both experimentally and, especially in the case of cervical cancer, epidemiologically. Both anal and cervical squamous cell carcinomas are preceded by precursor lesions called intraepithelial neoplasias that offer a chance of early detection. Given that cervical cytologic screening has led to a substantial reduction in the incidence of cervical cancer, the same approach should favorably impact the elevated incidence of HPV-related diseases of the anus in HIV patients. Reduced to these simple arguments, the rationale for anal cytology screening is straightforward and powerful. Furthermore, as cited in the review, it is buttressed by some clinical evidence. In this commentary, I wish to point out that in several ways the rationale for anal cancer screening with anal cytology and its implementation are not as firmly grounded as it may seem, and that overlooking these weaknesses may lead to unfortunate consequences and waste of resources to combat a real problem.
Making a parallel between the pathogenesis of HPV infections and diseases of the cervix and the anus has a sound justification as well as limitations. For example, both anatomic sites have a squamo-columnar junction, but only the cervix has a transformation zone defined by the area covered by the squamo-columnar junction as it recedes towards the os during lifetime. This process is under the influence of several factors, including hormonal. The use of oral contraceptive is a well-identified risk factor for the development of cervical cancer [1], but has not been reported to play any role in anal cancer. In HIV positive and negative women the prevalence of HPV DNA is higher in the anal canal than in the cervix [2], yet HPV-related anal diseases are not as prevalent as cervical diseases [3]. As further evidence that the anatomic location determines the specific pathogenesis of HPV infections, external genital warts are more common in HIV-infected than in HIV-negative men and women [4; 5], but recurrent respiratory papillomatosis, a disease that is felt to be associated with oral sex in adults [6], appears to be exceedingly rare in HIV-infected individuals. Nonetheless, the prevalence of HPV DNA in the oral cavity is higher in HIV seropositive than in seronegative subjects [7]. These observations suggest that if the general principles of cervical and anal cytology are similar, there are quantitative nuances whose magnitude and variability need to be well measured to accurately define the place of anal cytology in anal cancer screening.
To this end one would want to know on a population basis, not in the well-controlled and standardized settings of a few specialized academic centers, the natural history of untreated and treated anal HPV diseases in HIV patients. To date, as reviewed in the article and elsewhere [8], this knowledge is limited to patients whose treatment status is unknown. Moreover, with few exceptions the studies are cross-sectional rather than longitudinal [9].
The results of natural history studies of treated patients are fortunately becoming available, but much more is needed to address all the main questions [10; 11]. So far these studies, which are uncontrolled, focus on the treatment of anal high grade squamous intraepithelial lesions (HSIL). The results are encouraging, showing that once detected HSIL can be treated effectively. However, in addition to the absence of controls other than historical, other considerations mitigate the impact of these results. As with the cervix, anal cytology and histology have modest intra- and inter-observer reliability [12], this causes patient misclassification that reduces the accuracy of the measurement of the true treatment effect. Under-treatment and over-treatment are expected. Because the interventions and treatments are not free from financial, psychologic, and physical adverse effects [12], it is essential to know what happens to all patients subjected to anal cytology testing, not just those who qualify for a particular diagnosis and are treated.
Ultimately, one wants to know if these interventions reduce the incidence of anal cancer, evidence that is totally lacking. This will take time, and should not be an excuse for inaction. Indeed, HPV vaccination is now recommended without conclusive proof that it prevents cervical cancer, or any other HPV-related cancer. However, the expectation that it will be effective for these goals is firmly grounded on basic science and epidemiologic evidence. One should strive to approach this level of evidence for anal cytology.
Anal cytology is easy to obtain and does not offer any new interpretative difficulties for the cytologist accustomed to reading cervical smears. Issues of clinical effectiveness aside, moving anal cytology from the investigational domain to the realm of routine practice raises new substantial concerns. Cytologic abnormalities should prompt an evaluation by high-resolution anoscopy, which requires training and practice. Whether these practitioners do or will exist in sufficient number has not been addressed. We also do not know if the web-based educational tools put in place, or any other training for that matter, will be adequate to attain the results desired. It is at the last step of the intervention sequence, the treatment, that the guidelines are perhaps most fallible, because they lack validation. As discussed earlier, the best literature available so far is largely limited to the treatment of HSIL with the infrared coagulator. This does not mean that every proctologic operator will have this instrument available, or will accept for HSIL or any other HPV-related condition a uniform management approach. To date proctologic practices vary widely, and are largely informed by case-series, expert opinions, and personal experience, not an ideal basis for public policies. This variability of treatment mixed with the variability of diagnosis ensures that unnecessary and inappropriate treatments will occur. These are costs that have not been incorporated in otherwise favorable cost-effectiveness analyses of anal cytology screening [13].
Any reservations about the NYSDOH recommendations would be substantially diminished if the proper tools were in place to monitor their impact and correct promptly unavoidable errors. No doubt the number of detected anal precancers and cancers will increase. But this should not be the sole gauge of effectiveness of a public health intervention. The effects on morbidity could be difficult to measure accurately, and those on mortality will take time to document. Moreover, their significance might be difficult to analyze due to confounding variables such as sexual behavior and treatments. It will also be very difficult in the absence of controls to assess the magnitude of the unnecessary and harmful interventions, a critical information to capture not only to improve the guidelines, but also to measure the true costs of screening.
Sixty years after its introduction, few would doubt the success of cytologic screening for the prevention of cervical cancer. However, this approach was never submitted to a double-blind study showing its efficacy in reducing the morbidity and mortality of cervical cancer in women [14]. This lack of proper evidence no doubt contributed to disparate screening policies throughout the world [15], and explains why as of early 2000, there was still a need to prove the effectiveness of cervical cytology [16]. It would be regrettable if in the well-intended push to disseminate anal cytology without sufficient evidence and monitoring tools, one would ultimately delay the proper assessment of its indications, efficacy, mode of implementation, and cost-effectiveness.
William Bonnez, M.D.
Infectious Diseases Division, Department of Medicine
University of Rochester School of Medicine and Dentistry
Rochester, New York
References cited:
1. Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S, Goodhill A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet 2007;370(9599):1609-1621.
2. Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high risk HIV-negative women. J Infect Dis. 2001;183:383-391.
3. Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. JNCI. 2000;92:1500-1510.
4. Kiviat N, Rompalo A, Bowden R, Galloway D, Holmes KK, Corey L, et al. Anal human papillomavirus infection among human immunodeficiency virus-seropositive and -seronegative men. J Infect Dis. 1990;162:358-361.
5. Chirgwin KD, Feldman J, Augenbraun M, Landesman S, Minkoff H. Incidence of venereal warts in human immunodeficiency virus-infected and uninfected women. J Infect Dis. 1995;172(1):235-238.
6. Derkay CS, Darrow DH. Recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2006;115(1):1-11.
7. Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686-98.
8. Silverberg MJ, Abrams DI. AIDS-defining and non-AIDS-defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446-451.
9. Serraino D, Piselli P, Busnach G, Burra P, Citterio F, Arbustini E, et al. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. European J Cancer. 2007;43(14):2117-2123.
10. Stier EA, Goldstone SE, Berry JM, Panther LA, Jay N, Krown SE, et al. Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. J Acquir Immune Defic Syndr. 2008;47(1):56-61.
11. Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-Resolution Anoscopy Targeted Surgical Destruction of Anal High-Grade Squamous Intraepithelial Lesions: A Ten-Year Experience. Dis Colon Rectum. 2008 [online].
12. Colquhoun P, Nogueras JJ, Dipasquale B, Petras R, Wexner SD, Woodhouse S. Interobserver and intraobserver bias exists in the interpretation of anal dysplasia. Dis Colon Rectum 2003;46(10):1332-1336.
13. Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281(19):1822-1829.
14. Koss LG. Cervical (Pap) smear. Cancer 1993;71:1406-1412.
15. van Ballegooijen M, van den Akker-van Marle E, Patnick J, Lynge E, Arbyn M, Anttila A, et al. Overview of important cervical cancer screening process values in European Union (EU) countries, and tentative predictions of the corresponding effectiveness and cost-effectiveness. Eur J Cancer. 2000;36(17):2177-2188.
16. Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364(9430):249-256.
Reader Comment
Posted by cgonzalez on 10/20 at 04:46 PM
The decision on the part of the NYSDOH AIDS Institute to recommend annual anal Pap smear screening for certain members of our HIV infected citizenry was based on public health obligation. The risk for anal cancer, as has been noted in the excellent review by Drs. Bratcher and Palesky does not decrease with effective immune reconstitution with ARV therapy. The population of the HIV infected individuals in New York not only continues to grow but is aging as well. The Medical Care Criteria Committee, composed of some of New York State’s most eminent HIV treating physicians, used the following rationale in recommending to the AIDS Institute that screening for HPV associated anal malignancy be instituted.
-The risk of anal cancer in the MSM population (70/100,000).
-This is double the rate that prompted the recommendations for universal cervical screening of women.
-In the HIV+ MSM population the risk of anal cancer is reported as high as 144/100,000
-In the HIV+ female population, although the numbers are smaller, but the trends are similar.
-Universal cervical Pap screening for adult females was recommended in the United States in the 1960s decades before either the pathophysiology or oncogenesis of HPV was ever elucidated. HPV is unquestionably a cause of anal cancer.
-There has never been a randomized control trial for standard cervical Pap screening, nor would it be ethical to conduct such a study today.
-The efficacy of Pap screening in preventing cervical malignancies rests exclusively on decades of epidemiological data for validation.
-It is extremely unlikely that a 10-year clinical trial demonstrating the efficacy of anal Pap smears as a screening method in any HIV-infected population will ever be performed.
-And lastly, HPV-initiated carcinomas are clearly preventable malignancies.
As a general screening method, Pap smears although they have a limited specificity, have the advantage of over 75 years of clinical familiarity and are part of routine laboratory pathology department protocols. These screening tests allow, if nothing else, an alert to the medical provider that a potential malignant lesion may be present and monitoring (and if necessary treatment) is appropriate. What the appropriate intervention may be, whether infra-red coagulator treatment or careful clinical “watchful waiting” merits clinical trial examination. Not screening however, does not negate the oncogenic potential of HPV, only the effective ability of an early intervention.
Charles J. Gonzalez, MD
NYS DOH AIDS Institute
Reader Comment
Posted by eganz on 10/21 at 10:18 PM
I want to thank the authors for an excellent and informative review of anal HPV and AIN. I also want to thank those who gave very insightful comments. I think we all agree that although AIN management and treatment still deserves more intensive clinical research in its efficacy in treatment and management, we also agree that waiting for a complete evaluation of its efficacy is impractical and unethical knowing what we know from CIN. So where do we go from here? William Bonnez, M.D. in his comment above addresses a very important health care issue: creating an infrastructure for public health care management of diagnosis AND treatment of AIN. One without the other would be unacceptable. Although diagnosis may be feasible with anal pap smears followed by High Resolution Anoscopy… “It is at the last step of the intervention sequence, the treatment, that the guidelines are perhaps most fallible, because they lack validation”, states Dr. Bonnez above. I agree. In the past 60 years of CIN screening, diagnosis and treatment, The American College of Obstetrics and Gynecology have taken an active effort in creating this infrastructure in education at all levels of the disease. Not all colorectal surgeons are “on board” with the AIN screening and treatment. It may take time to have the colorectal surgery community join us on this very important public health care issue. I personally feel that this disease (AIN) and its screening deserves the attention of the colorectal community at all levels.
Eric M .Ganz, MD, FACOG
Department of Obstetrics and Gynecology
Comprehensive Care Center
St. Luke’s - Roosevelt Hospital Center
New York, NY
Author Comment
Posted by Joel Palefsky on 11/01 at 05:53 PM
I would like to thank all of those who have commented as of the time of this posting (10/31/08) for their thoughtful comments. I think we are all in agreement with the following: 1. AIN and anal cancer are growing problems that should not be ignored; 2. Anal screening and treatment programs should be guided by the best data possible and by years of experience with screening for cervical disease; 3. It will take some time to amass the data that we all want, and there is controversy as to how best to proceed until we have those data; 4. Until we have those data everyone should at a minimum have an annual digital rectal exam to screen for anal cancer; screening could be done in those clinical settings where the entire range of clinical expertise needed to screen, diagnose, treat and follow patients with AIN is available. Ideally data will be collected in those settings to better inform these screening programs. Clinicians at those sites should train their colleagues locally and elsewhere to increase the critical mass of clinicians trained to perform screening, diagnosis and treatment of HRA nationwide; and 5. Quality assurance procedures at each step of the process will need to be implemented to ensure that we are providing patients with the best service possible.
Joel Palefsky, M.D., F.R.C.P.(C)
Professor of Medicine
Associate Dean for Clinical and Translational Research
UCSF
Reader Comment
Posted by Michael Berry on 05/31 at 02:27 PM
If you are interested in learning high-resolution anoscopy (HRA), the UCSF Anal Neoplasia Group recommends that prospective trainees take a basic colposcopy course to understand the fundamentals of colposcopy and lesion recognition, a didactic course in HRA, and then a preceptorship observing the performance of HRA in an established anal neoplasia clinic. Direct ?hands-on? experience performing many HRA exams is necessary to become proficient. This may take a number of years based on the volume of patients that a provider sees and whether the patients have a high likelihood of disease.
Standards for training and proficiency in HRA have yet to be clearly defined. As an example, colposcopy training programs require 25-50 proctored examinations including at least 3 high-grade cervical cases and at UCSF it is 100. Experienced colposcopists find that learning HRA can be challenging; visualizing anal lesions is more difficult that performing cervical colposcopy. For the AIDS Malignancy Consortium HPV Working Group, the following standards for proficiency were determined. Examiners are asked to submit logs of 50 consecutive exams performed in HIV-positive patients listing cytology and histology. At least 30% of subjects should have high-grade neoplasia as the most severe histology per patient. The level of 30% was chosen to represent what is felt to be a low estimate of the actual prevalence of HSIL in HIV-positive patients. Providers are certified after a final visit in which an expert in HRA directly observes 10 HRA exams.
There are two basic colposcopy courses offered by the American Society of Colposcopy and Cervical Pathology (www.ASCCP.org) in the upcoming year listed as Comprehensive Colposcopy:
July 30-August 2, 2009 in Providence, RI
October 7-10, 2009 in Salt Lake City, UT
Two courses in high-resolution anoscopy will be offered by the ASCCP:
December 12-13, 2009 in Naples, FL (this is not listed on the ASCCP website as of 6/1/09 and will be associated with an Advanced Colposcopy of the Lower Genital Tract Course, you may call 800-787-7227 to be placed on a waiting list)
August 12-15, 2010 in San Francisco, CA (HRA will be available as an optional track during a Comprehensive Colposcopy Course)
J. Michael Berry, MD
Associate Clinical Professor of Medicine
UCSF
You must be logged in to post a comment. Login | Register