In January of 2005, the United States Centers for Disease Control and Prevention released its revised guidelines for nonoccupational postexposure prophylaxis (PEP) of HIV transmission for high-risk sexual contacts. These guidelines have raised many questions about the evidence supporting PEP and the ways in which it is best provided to patients in clinical settings. The idea of using antiretrovirals to prevent transmission is not new, since guidelines for occupational exposures have existed since 1996. For sexual transmission, prophylaxis with antiretrovirals before a potential exposure—pre-exposure prophylaxis (PrEP)—or after the exposure occurs (PEP) is controversial and often leaves clinicians unsure of how to provide the best care to their at-risk patients.
In March of 2006, Michelle Roland, MD, and Angela Kashuba, PharmD, were invited to a PRN meeting to discuss this complex issue. Dr. Roland discussed the challenges and opportunities she has experienced in PEP research, particularly focusing on the broader context of nonpharmacologic interventions that prevent transmission, and the current state of PEP in the United States and internationally. Dr. Kashuba focused on work being done to determine which antiretrovirals are optimal for PrEP and PEP applications, and to provide a rational framework for further policy making.
| Introduction to Sexual Transmission of HIV and Antiretrovirals | Top of page |
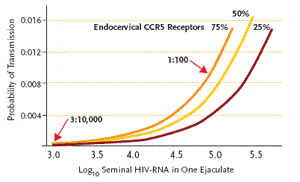
Figure 1. Estimated male-to-female per-sexual contact HIV-1 transmission
probability for different seminal viral load and for different receptor cell counts when 100% of the isolates in the semen are non-syncytiuminducing (nsi). The horizontal axis represents log10 seminal viral load in one ejaculate and the vertical axis represents the male-to-female per-sexual contact hiv-1 transmission probability. Three different lines represent different receptor cells/mm2 counts; 25th percentile, 50th percentile, and 75th percentile.
Reproduced with permission from Chakraborty H, et al. aids. 2001;15:621-627.
Dr. Kashuba provided data supporting the use of PrEP and PEP. Endemic spreads of infection are a function of the size of the inoculum at the site of infection, the contagiousness of the organism, and the duration of infection (Anderson, 1991). In HIV transmission, the size of the inoculum corresponds to the viral burden. For sexual transmission, this specifically applies to the viral load in the genital tract.
In a model of sexual transmission, an Emory University team calculated that male-to-female transmission may vary from 1:100 to 3:10,000 incidences per sex act, depending on the viral load in semen and the number of endocervical CCR5 receptors in the woman (see Figure 1) (Chakraborty, 2001). Adding to this picture of transmission is acute HIV infection, where viral load is very high, and patients may not be aware of the diagnosis and thus be unable to make behavioral changes. The rate of transmission during this time period has been estimated at 1:30 to 1:200 per sex act, depending on the type of exposure (Cohen, 2005). However, as Dr. Kashuba explained, patients all along the continuum of infection contribute to transmission, since HIV viral load increases with concomitant sexually transmitted diseases (eg, syphilis) and increases during late-stage HIV disease. The majority of HIV-infected patients are in the asymptomatic, or drug-controlled, phase of disease, and the number of patients in this group may considerably add to the rate of transmission.
In several studies involving men and women, antiretroviral therapy has been shown to decrease HIV-RNA in the genital tract of an infected individual (Pereira, 1999; Vernazza, 2000; Dumond, 2006). Taken together with the success of occupational PEP and the prevention of mother-to-child transmission, these data provide biologic plausibility for the use of antiretrovirals to prevent nonoccupational HIV transmission.
| Controversies with PEP Guidelines | Top of page |
Although most clinicians and researchers seem to agree that PEP is likely to be a valuable tool in preventing HIV infection, there is little agreement about who should receive antiretrovirals based on the source of the exposure, which drugs and how many should be provided, when they should receive them following an exposure, and what type of follow-up care should be offered. Dr. Roland highlighted these issues, where guidelines vary considerably from state to state and country to country. Time to initiation, the HIV status of the exposure source, the number and type of antiretrovirals to use, and follow-up monitoring recommendations are detailed below for guidelines produced by the CDC, New York, California, and Rhode Island (see Table 1).
The state of New York is the only guideline-issuing body to limit the use of PEP to the first 36 hours after exposure. It is widely believed that the sooner a PEP regimen is started postexposure, the greater the chances that it will prevent HIV infection. This, as highlighted above, has been demonstrated in animal models. Thus, even though PEP is often offered for up to 72 hours after exposure, it should be initiated as early as possible. After 72 hours, PEP is not effective, and there are gradations in efficacy from 24 hours postexposure, to 36, 48, and 72 hours. This is a complicated message to communicate: most will hear that PEP can be initiated up to 72 hours postexposure, but the true message is that PEP needs to be started as soon as possible for greatest efficacy, and should not be started more than 36 or 72 hours postexposure, depending on the guidelines. Dr. Roland offered her message for trying to decide whether to initiate PEP: “If you’re feeling ambivalent about this, we can always go ahead and start it and then you can stop it. It’s easy to stop. But you can’t even have it started if you decide in two days that this is what you want to do.”
The prevalence of HIV infection in the local community is important when deciding if treatment is warranted after an exposure from a source with an “unknown” HIV status. For example, in the gay male population in San Francisco, the prevalence of HIV infection is approximately 30% to 40%. Therefore, says Dr. Roland, any MSM exposure to an “unknown” source should be considered high risk. In areas with a lower prevalence, the decision may not be as clear. In contrast to occupational exposures, rarely, if ever, is the source available for testing. Therefore, applying local demographic information to national recommendations, such as those produced by the CDC, which definitively recommend PEP for known HIV positive exposures and case-by-case judgment for all “unknown” status exposures, is critical for the clinician. The CDC guidelines should not be applied so that PEP is provided only to those with exposures to known infected sources, particularly considering that roughly 25% of infected adults in the U.S. are unaware of their diagnosis.
The guidelines differ as to how many, and which, antiretrovirals are to be used in a PEP regimen. More aggressive three-drug PEP regimens are warranted with a known HIV-infected source harboring drug-resistant virus. In turn, the local viral susceptibility patterns and types of resistance seen in the community may be an important consideration for the clinician when deciding which agents to choose.
Tenofovir in combination with coformulated zidovudine and lamivudine (Combivir) is recommended in the New York guidelines. The CDC and Rhode Island both recommend three drugs, including a protease inhibitor or efavirenz, but differ on the specific agent recommended. Two drugs are recommended in California, unless the antiretroviral history and resistance patterns of the source are known. If that information is available, more than two agents may be required. The drug combination that Dr. Roland is currently looking toward using is tenofovir coformulated with emtricitabine (Truvada), which has promising results in animal studies and is generally well tolerated.
Tolerability of the chosen regimen is an important consideration. Research presented at the 13th CROI suggests that the protease inhibitor–based regimen recommended by the CDC—lopinavir/ritonavir (Kaletra) plus Combivir—may be prematurely discontinued in approximately 25% of patients due to adverse effects (Rabaud, 2006). Alternate regimens had high discontinuation rates as well, ranging from 19% for tenofovir plus zidovudine/lamivudine to 35% for a nelfinavir (Viracept)-based regimen.
Although all guidelines recommend an HIV test at baseline, and again during follow-up, other laboratory monitoring recommendations vary. Generally, PEP regimens last 28 days, and the question of which adverse effects might be expected from the antiretrovirals in the regimen likely can help guide patient care. Dr. Roland’s experience in California has been that patients rarely have laboratory abnormalities, and she does not recommend extensive lab follow-up. However, other guidelines, such as those produced by the CDC and New York State, do recommend several screening lab tests at baseline, during treatment, and four to six weeks posttreatment.
| PEP and Sexual Assault in Africa | Top of page |
Providing PEP after a sexual assault is of great interest to providers, both in the U.S. and other countries. Dr. Roland shared some of her experiences providing PEP services to women in Cape Town, South Africa, at a comprehensive rape treatment center. Sexual assault in the context of HIV prevention exposes several links between violence and women’s ability to protect themselves against HIV exposures. PEP has provided healthcare workers a means of entering this highly politicized, uncomfortable arena that previously has been considered a psychosocial issue. Although this was a unique experience, many parallels between the provision of PEP in Cape Town and San Francisco were observed.
A challenge highlighted by Dr. Roland is that the literature regarding the provision of post-assault PEP is only from North America and Europe. This body of literature suggests that sexual assault survivors have a very low uptake of PEP and extremely low completion and follow-up rates. It is unclear whether this is a function of healthcare providers not offering or recommending PEP, or if it is a function of the survivor’s rational decision-making process. Exposure to HIV in a very low prevalence setting may influence the survivor to decline PEP. Alternatively, it may be the result of an extraordinarily traumatic experience that impairs the decision-making process. Adherence is a challenge and currently, it is not known whether adverse effects of the drugs or symptoms of acute stress disorder as a result of the assault are contributing factors to nonadherence.
Dr. Roland shared data from 335 mostly young women participating in this study in South Africa. Although overall completion rates are relatively high, with the median completion rate being 27 out of 28 days of PEP, four-day recall surveys reveal that doses are often missed. In fact, up to one-third of patients either discontinued or missed at least one dose in the preceding four days. Although nonadherence was initially attributed to adverse effects, shame, and being emotionally overwhelmed because of pill burden, in many cases, it was simply because patients forgot, or they were away from home and did not have their medications with them. This is similar to the adherence barriers of patients in typical clinical situations. In analyzing the adherence data, no predictors of nonadherence were found. This serves to reinforce the notion that every patient should be provided with adherence counseling to enhance the rates of adherence in a population that is not “sick” and may not as fully understand the consequences of missed doses as they would if they had physical signs and symptoms of their disease. Very similar results in adherence and discontinuation were seen in the two populations of Cape Town and San Francisco, with only 79% of patients from San Francisco reporting having taken all of their medications perfectly in the preceding four days.
Another recurring theme previously seen in the San Franciscan MSM population is that of ongoing exposures. More than half of the 135 women seen by Dr. Roland were having unprotected sex prior to their rape, and most did not know the HIV status of their partner(s). This is a previously overlooked aspect of providing PEP to assault survivors: the consideration of what was occurring in their lives apart from the assault. One-third of the subjects were having unprotected sex one month after their assault. After three months, one-half were having unprotected sex, and after six months, two-thirds were having unprotected sex. Controlling for other variables, the only predictor for having unprotected sex after the assault was having unprotected sex prior to the assault. This highlights the need and responsibility to conduct a thorough behavioral risk assessment and to provide counseling to reduce future risk in addition to providing medications. Dr. Roland noted that 4/135 women in this cohort followed for six months seroconverted, two of whom were probably true PEP failures (one due to poor adherence) and two of whom had other exposures that may have resulted in their infections. The contribution of ongoing exposures resulting in seroconversion in both of these populations is the reason that provision of behavioral counseling should be an integral part of PEP.
In this study, the issues of the resources needed to provide PEP in postassault settings was explored. The results indicate that pro-active follow-up is needed, and Dr. Roland’s group accomplished this by hiring nurses to provide home visits, field telephone calls, and provide very proactive follow up rather than passively waiting for patients to come of their own accord. These nurses made 161 tracing attempts for 135 people to provide follow-up care. Several referrals were also made for outside rape counseling services. Overall, this experience showed that providing services such as this one can be quite resource-intensive.
At the international level, the issue of resources is being explored by the WHO. Some of the key issues identified include the fact that PEP services have to be provided as part of a comprehensive prevention package and, generally, should be integrated into existing services rather than developed on their own. Optimal service delivery sites have to be identified in each setting as well, which has clearly been the case in the United States. Dr. Roland’s experiences in different systems have shown that, “every system has come up with a different solution based on their unique strengths and opportunities and needs within their community about how and where to provide PEP. Clients must receive appropriate information about the risks and benefits of PEP in order to provide informed consent.” Adherence, adverse effect, risk-reduction, and trauma counseling, and available clinical follow up services to provide adverse effect management and follow-up HIV testing need to be part of the PEP package, as do pregnancy testing and emergency contraception.
| Ongoing Clinical Research of PrEP | Top of page |
Although several trials of PrEP have been discontinued for various reasons, clinical research in this area is ongoing. One trial, sponsored by the CDC and being conducted in Atlanta and San Francisco, is designed to assess the renal and bone safety profile of tenofovir in the MSM population. An additional safety trial of tenofovir in high-risk women is ongoing in Ghana. Each of these trials will evaluate 400 subjects. Several other trials to assess both the safety and efficacy of tenofovir are ongoing in Thailand (IV drug users), Botswana (high-risk subjects), and Peru (high-risk men). In total, these various safety and efficacy trials will enroll approximately 4200 high-risk subjects.
| Conclusion | Top of page |
With PrEP and PEP come several unique challenges and opportunities. A large body of behavioral, clinical, and animal research is in development in an effort to best care for patients in need of prophylactic treatment for HIV exposures. For the practicing clinician, particularly those with high-risk patients, understanding the current literature, treatment guidelines, and local infection prevalence and drug-resistance epidemiology should assist in selecting appropriate prophylactic regimens for patients. Providing risk-reduction, adherence, and adverse event counseling is also critical in this effort.
| References | Top of page |
