The first question to ask in attempting to understand the pathogenesis of HIV infection is not how the virus causes disease, but why. “In its natural host,” Dr. Daniel Douek stated, “HIV does not cause disease. SIV in old-world monkeys and chimpanzees in Africa does not cause disease. These animals have very high viral loads, but none of them progress to AIDS. So why does the same virus, when you put it into humans, cause disease at all? No other infection in humans, whether it’s acute or chronic, causes such a profound and inevitable depletion of CD4+ cells.”
To answer this question, scientists have attempted to shed light on how HIV infection causes CD4+ cell depletion in humans. It is universally accepted that HIV typically, but not always, runs a predictable course in the human body. During acute HIV infection, there’s a very high viral load. Around the time of seroconversion, the viral load usually decreases and ushers in the chronic phase of infection, which lasts approximately ten years until the onset of AIDS. As for the CD4+ cell count, there’s a transient dip during the acute phase of infection, followed by a slow, steady drop during the chronic phase of infection that ultimately renders the immune system compromised.
In 1995, Dr. Ho and colleagues shed a considerable amount of light on the kinetics of HIV and its major target, the CD4+ cell. Using the protease inhibitor ritonavir, Dr. Ho’s team determined that the rapid reduction of free virus in blood plasma—which occurred within a few days of drug therapy—was associated with a significant increase in circulating CD4+ cells. However, as drug-resistant virus evolved, there was a considerable increase in viral load and a decrease in CD4+ cells (Ho, 1995; Wei, 1995). As simple as this chain of events sounds, it was precisely these changes in free virus and CD4+ cells that permitted mathematical modeling of the kinetics of HIV replication and CD4+ cell depletion and turnover rates (Perelson, 1996).
Such mathematical models allowed for the calculation of the half-life of cell-free HIV and infected CD4+ cells, as well as the total body burden of HIV. This kinetic model predicted, among other things, that in HIV-infected individuals the half-life of CD4+ cells is shortened and that the rate of production of CD4+ cells is very high.
“The model predicted that there is a lot of CD4+ cell death and a lot of CD4+ cell production,” Dr. Douek said. “This gave us a much clearer picture of the pathogenesis of HIV. It made perfect sense. The model of pathogenesis was that the virus was infecting and killing CD4+ cells, while there was ongoing production of new CD4+ cells. However, the production simply isn’t enough. So over the period of ten years, all of the CD4+ cells are eventually lost.”
This model has colloquially been referred to as the tap-and-drain model. In this model, water in a sink represents CD4+ cells, an unplugged drain represents HIV infection, and water flowing from the tap represents the production of new CD4+ cells. “With the tap and drain model, the tap—the production of new CD4+ cells—is simply not powerful enough to keep up with the flow of water going down the drain—the number of HIV-infected CD4+ cells,” Dr. Douek said. “Over time, the source of the water eventually runs out, leaving an empty sink, which is synonymous with AIDS.”
Some of Dr. Douek’s early experience in the lab helped to further the tap-and-drain model. “We found that HIV infection causes a state of high turnover,” he said. “But we have also shown that HIV infection is actually characterized by chronic immune activation, where there is an increased amount of cell death and proliferation of CD4+ cells, not increased thymus-derived production of new CD4+ cells.”
In recent years, however, the entire tap-and-drain model has been questioned, most notably by immunologists. The debate essentially boils down to two diametrically opposed questions. The first question fits with the older idea of pathogenesis: Does HIV cause massive CD4+ cell death, with increased immune activation being the homeostatic response? In other words, is it because HIV is a virus that attacks and kills CD4+ cells that these cells become depleted? And is the homeostatic response to the death of the CD4+ cells responsible for replacement? “If you give someone chemotherapy and you deplete the immune system, there’s a homeostatic response to replace the cells,” Dr. Douek explained. “This reflects the tap-and-drain model.”
The second question regarding the pathogenesis of HIV reflects more recent thinking by various research teams. Does HIV cause massive immune activation with increased CD4+ cell death being the natural consequence? “Again, with HIV being a virus capable of causing massive immune activation, is it possible that immune activation—not the virus—is responsible for the death of CD4+ cells?”
There have been several observations to support this hypothesis. “First of all,” Dr. Douek pointed out, "there is no disease in natural SIV infection, despite the presence of very, very high viral loads—viral loads that are often higher than in humans infected with HIV. In the natural hosts, SIV doesn’t appear to be doing anything, despite active replication.” Another factor to consider is that the frequency of HIV-infected CD4+ cells is extremely low. “Approximately .01% to 1% of peripheral blood CD4+ cells are infected with the virus. It’s very low. So how could the virus be responsible for killing all the CD4+ cells when it doesn’t infect very many of them?” He also stressed that infected CD4+ cells are activated, so they are destined to die anyway. “As a part of our laboratory training, we were taught that in order to get the CD4+ cells infected, you have to activate them in vitro. Activated cells are going to die anyway, so it doesn’t matter if HIV infects them.” It has been observed that both CD4+ and CD8+ cells have increased proliferation and death in HIV infection. “Despite the high turnover of both types of cells, only the CD4+ cells decline.” Finally, suppression of HIV using antiretroviral therapy does not immediately change the high death rates of CD4+ cells. “This also suggests that HIV is not directly responsible for the death of CD4+ cells,” Dr. Douek said.
Based on these observations, a new mechanism of HIV disease progression was born: that the increased CD4+ and CD8+ cell death and proliferation is a consequence of virus-induced immune activation, not virus-mediated killing. “This process is gradual and protracted and eventually leads to AIDS,” Dr. Douek said. “However, this still doesn’t explain why CD4+ cells are depleted in HIV infection. Perhaps the answer involves mechanisms raised in both HIV pathogenesis models.”
| Acute HIV Infection | Top of page |
In a paper published in 1998, Dr. Ronald Veazey and his colleagues demonstrated in the macaque SIV model that the earliest targets of infection are mucosal CCR5+ memory CD4+ cells, whatever the route of infection (Veazey, 1998). SIV infection resulted in profound and selective depletion of CD4+ cells in the intestine within days of infection, before any such changes in peripheral lymphoid tissues. The loss of CD4+ cells in the intestine occurred coincident with productive infection of large numbers of mononuclear cells at this site.
To better understand the mechanism underlying the depletion of memory CD4+ cells, a team of investigators that included Dr. Douek longitudinally sampled blood, lymph nodes (mesenteric and inguinal), and mucosal tissues (jejunum) from macaques before and after SIV infection at a regular and high frequency (Mattapallil, 2005). This allowed the investigators to address the issue of tissue distribution of CD4+ cells, a matter that clouds the interpretation of measurements taken solely from peripheral blood. The study team also performed highly sensitive PCR analyses to determine which subsets of CD4+ cells were infected, and to what extent the virus propagated through these subsets (discussed in greater detail below).
The loss of memory CD4+ cells was explained by massive infection of the cells by SIV. Specifically, 30% to 60% of memory CD4+ cells through the body are infected by SIV around the peak of infection, and most of these infected cells disappear within four days. The data also demonstrated that the depletion of memory CD4+ cells occurs to a similar extent in all tissues (see Figure 1). As a consequence, over one-half of all memory CD4+ cells in SIV-infected macaques are destroyed directly by viral infection during the acute phase, an insult that is followed by immune deficiency.
Figure 1.SIV Infection and CD4+ Cell Depletion
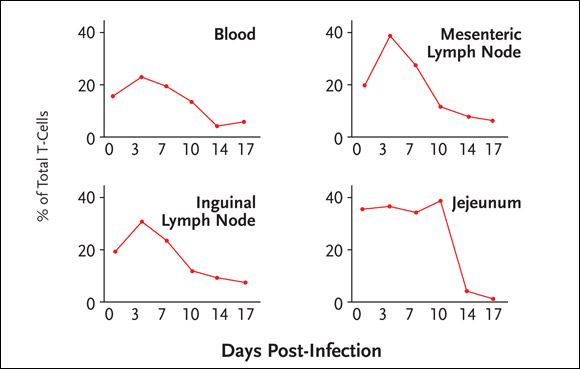
In SIV-infected macaques, 30% to 60% of memory CD4+ cells throughout the body are infected by SIV around the peak of infection, with most infected cells disappearing within four days. The depletion of memory CD4+ cells occurs to a similar extent in all tissues.
Working with investigators at the University of Minnesota and the Mayo Clinic, Dr. Douek has been studying CD4+ cell depletion in HIV-negative and HIV-positive human subjects. Blood, lymph node biopsies, and ileal biopsies are being collected from the patients. HIV-positive patients have been providing samples at baseline and after the commencement of antiretroviral therapy.
“If you look in a healthy individual,” Dr. Douek explained, “there is an abundant supply of CCR5+ CD4+ cells in the gut through which HIV can rapidly propagate. It’s important to remember that HIV probably needs a continuum of cellular targets in order to propagate, so the infectious unit is not the free virion but rather the HIV-infected cell.”
To determine if there is preferential depletion of CD4+ cells in the guts of HIV-infected patients, Dr. Douek’s group has looked at the percentage of T-cells expressing CD4 in the gut, blood, and lymph nodes (Brenchley, 2004). “We found that there were lower percentages of CD4+ cells in the gut of HIV-positive patients (see Figure 2). Researchers have done this before, simply looking at percentages. And just looking at these percentages, it looks as if there’s preferential depletion in the HIV-positive gut, compared to other compartments. However, we also saw lower percentages of CD4+ cells in the guts of our HIV-negative patients. In other words, there was nothing to be learned here, given that it looks as if there’s preferential depletion in HIV-negative individuals, which simply doesn’t make sense.”
Figure 2.HIV Infection and Preferential Depletion of Gut CD4+ Cells
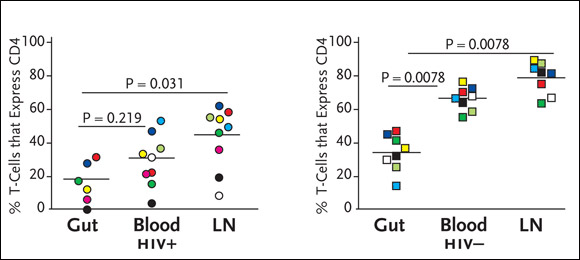
The graph on the left shows percentages of CD4+ cells in the GI tract, peripheral blood, and lymph nodes (LNs) in HIV-positive individuals, compared to percentages of CD4+ cells in the same compartments in HIV-negative individuals. While the GI tract contains significantly lower percentages of CD4+ cells in both HIV-positive and HIV-negative individuals, there is still evidence of extensive depletion of particular CD4+ cell subsets, including those specifically targeted by HIV in the GI tract.
The key, Dr. Douek argued, is to look for CCR5+ T-cells in the gut. In HIV-uninfected individuals, virtually all CD8+ cells in the gut expressed CCR5, whereas more than half of the gut CD4+ cells expressed CCR5. In contrast, only 5% to 10% of lymph node-derived CD4+ and CD8+ cells and 10% to 30% of peripheral blood-derived CD4+ and CD8+ cells expressed CCR5. In an HIV-infected, treatment-naïve individual—and in four other HIV-infected individuals—there was preferential and substantial depletion of gut-derived CCR5+ CD4+ cells, consistent with the Mattapallil SIV data discussed above. This depletion was specific to CD4+ cells, as all gut-derived CD8+ cells maintained CCR5 expression. Importantly, the nearly complete depletion of CCR5+ CD4+ cells was restricted to the gut. The percentage of CCR5+ CD4+ cells in lymph nodes and peripheral blood from the HIV-infected individuals did not differ from those in the HIV-uninfected individuals. This was surprising, as immune activation, altered trafficking, and proliferation per se should result in accumulation of CCR5+ CD4+ and CD8+ cells in the peripheral blood, lymph nodes, and gut. Although this was clearly the case for the CD8+ cells—“there was a massive expansion of CD8+ cell that express CCR5, which makes sense,” Dr. Douek said—CCR5+ CD4+ cells did not appear to be elevated.
“Based on these data,” Dr. Douek commented, “we understand where depletion occurs—memory CD4+ cells, mostly in the gut and in the mucosa. We also understand how much depletion occurs. It’s the majority of memory CD4+ cells. What we still want to know is if the virus is directly responsible for the depletion of CD4+ cells. We also want to know the frequency of infection of memory CD4+ cells, which is what we set out to determine.”
An image from an endoscopic analysis of the terminal ileum in a patient with newly acquired HIV infection, compared to that of an HIV-negative control, is provided in Figure 3. In healthy HIV-uninfected individuals, the typical gross anatomical appearance of the terminal ileum shows large lymphoid aggregates. In HIV-positive individuals—including those newly infected with the virus—the terminal ileum is striking in its almost complete absence of discernible lymphoid tissue.
Figure 3.Which is Normal? Guess Again.
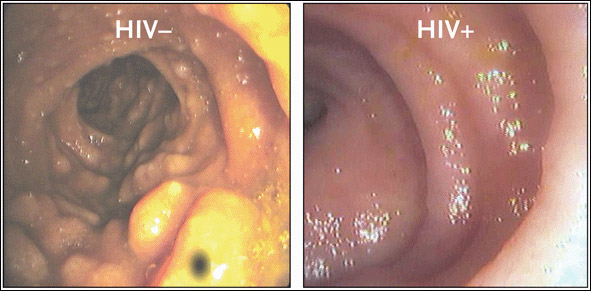
Endoscopic photographs from an HIV-uninfected, healthy individual (left) and an individual newly infected with HIV (right). In the HIV-uninfected individual, the gross anatomical appearance of the terminal ileum shows large lymphoid aggregates. In the newly infected HIV-positive individual, the terminal ileum is striking in its almost complete absence of discernible lymphoid tissue, suggestive of substantial CD4+ cell depletion in the GI tract at a very early stage of HIV infection.
Source:Brenchley, 2004. Journal of Experimental Medicine 200(6):752. Adapted with permission of The Rockefeller University Press.
In order to understand the role of viral infection of CD4+ cells on their dynamics, Drs. Mattapallil, Douek, and their colleagues quantified both plasma and cell-associated virus in macaques (Mattapallil, 2005). They used a highly sensitive quantitative PCR for SIV gag on bulk-sorted subsets of T-cells to determine the extent to which direct SIV infection could account for the loss of memory CD4+ cells. Starting shortly after infection, the number of SIV copies in memory CD4+ cells subsets rose steadily, peaking at day 10 after infection in all tissues examined. At peak, the study team observed a very high number of SIV copies (50 to 200,000 per 105 memory CD4+ cells).
“What we needed to determine was the reason for the high levels of SIV,” Dr. Douek explained. The high levels might be due to a very high number of copies in a small number of cells, or they might be due to a low number of copies in nearly all memory cells. To distinguish between these possibilities, the research team quantified the amount of SIV-DNA in single-sorted memory T-cells, including PBMCs, along with those in mesenteric lymph nodes, inguinal lymph nodes, and the jejunum. They determined that memory CD4+ cells in all the tissues carried, on average, 1.5 copies of HIV-DNA. This measurement is in agreement with published data in humans, where infected CD4+ cells in lymph nodes carried, on average, two copies of viral DNA. From the quantification of SIV at the single-cell level, it was determined that the fraction of cells infected in the different tissues ranged from 30% to 60% of all memory CD4+ cells at the peak of infection (see Table 1).
| Table 1. Quantification of SIV by Single Cell qPCR | ||
| Tissue | Gag copies/cell | Infected fraction of Memory CD4+ cells |
| PBMC | 1.45 | 30% |
| Mesenteric lymph node | 1.51 | 54% |
| Inguinal lymph node | 1.45 | 49% |
| Jejunum | 1.61 | 60% |
In concluding this part of his discussion, Dr. Douek stressed that acute HIV infection is a completely different disease from chronic HIV infection. “For years, we’ve been looking at chronic infection and basing all of our conclusions on what we saw during chronic infection, which is simply the wrong time and the wrong place to fully understand the pathogenesis of HIV,” he said. During acute HIV infection, the primary target is the total memory CD4+ cell compartment. The targeted CD4+ cells are mostly in the mucosa, which account for the majority of the CD4+ cells in the body. “Approximately 60%, if not higher, of all the memory CD4+ cells are infected in the acute phase of the infection,” he said. This rate is at least a hundredfold or a thousandfold higher than in peripheral blood in chronic HIV infection.” By day 14 after infection is established, 80% of infected cells have disappeared. In other words, the majority of memory CD4+ cell depletion occurs during the acute phase of infection. The loss of memory CD4+ cells can solely be ascribed to the consequences of viral infection. “You don’t necessarily have to evoke any other mechanisms,” he said.
Patients undergoing chemotherapy or receiving immune-suppressive therapies after a transplant are prime examples of what is seen during acute HIV infection. “In my lab we do a lot of work with people with leukemia and immune reconstitution,” Dr. Douek explained. “What we see in these patients gives us a clue as to why CD4+ cells don’t come back in HIV infection, whereas the CD8+ count is fine.” After a transplant, CD8+ cells rapidly return to normal levels, whereas the CD4+ cell count rarely returns to normal levels. “There’s a physiologic limitation on CD4+ cells—on maintenance of the CD4+ cell pool after massive depletion from chemotherapy or the effects of HIV infection.” For starters, CD4+ cell reconstitution is limited and is age dependent. Second, it depends on naïve CD4+ cell reconstitution and intact lymph nodes. Third, naïve CD4+ cell reconstitution depends on thymic output. Finally, the CD8+ cell pool appears to maintain itself adequately by expansion. “This is all after chemotherapy is completed, “Dr Douek said. “After chemotherapy, people do not progress to AIDS, whereas people with HIV do. So what’s the difference? The difference is between chemotherapy, which is halted, and the virus, which isn’t. The mechanisms that occur during chronic HIV infection essentially finish what is started.”
| References | Top of page |
