The successful use of highly active antiretroviral therapy (HAART) can dramatically suppress human immunodeficiency virus (HIV)-1 viral replication and effect significant immune reconstitution.1–4 However, despite full access to antiretroviral agents, the emergence of antiretroviral-resistant HIV-1 strains and/or drug toxicities can derail effective treatment. A prospective study of patients in a New York City cohort with acute and early HIV-1 infection found the prevalence of transmitted resistance to at least one antiretroviral agent to be 24.1%.5 Consequently, the need to develop antiretroviral agents with novel mechanisms of action persists for the treatment of both antiretroviral-experienced and antiretroviral-naïve patients. In October 2007, the United States Food and Drug Administration (FDA) approved the first drug in the integrase-inhibitor class for the treatment of HIV-1 as part of combination antiretroviral therapy in treatment-experienced patients, adding to the available chemotherapeutic agents for the effective treatment of HIV/AIDS.
| HIV Integrase and Integration | Top of page |
Successful HIV-1 replication requires the use of 3 enzymes: reverse transcriptase, integrase, and protease. The HIV-1 life cycle initiates with viral entry into host immune cells that express surface CD4.6–9 After viral entry, HIV-1 reverse transcriptase converts its single-stranded RNA into double-stranded DNA (dsDNA), at which time integrase assembles in a stable complex with viral DNA—the pre-integration complex—and is chaperoned into the nucleus.10 Subsequent integration of HIV-1–complementary DNA (cDNA) into the host genome is a two-step process catalyzed by the HIV-1 integrase enzyme (Figure 1). Initially, 2 nucleotides are excised from the 3´ ends of the nascent HIV-1 DNA. This is followed by the irreversible, covalent insertion of HIV-1 viral genomic DNA into the host chromosome.11,12 While the HIV-1 virus is known to preferentially target sites within transcribed host genes for integration—so called “hot spots”—the factors underlying these preferences are not entirely clear.13,14 When integrase is inhibited, host enzymes circularize the viral cDNA, and 2-long terminal repeat (LTR) circles accumulate in the nucleus.15–18 Inhibiting integrase from performing its essential functions therefore blocks stable integration of HIV-1 DNA into the host genome and prohibits the establishment of viral latency within the host cell, preventing high-level HIV-1 replication and infection of new cells by competent virus.19
Figure 1. Integration of HIV-1–cDNA into host genome.
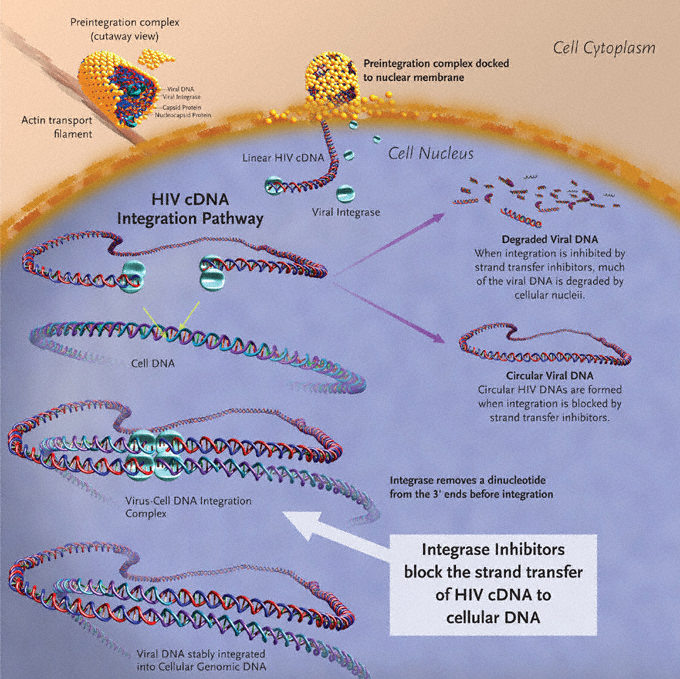
Image created by Louis E Henderson, PhD.
| Clinical Trials | Top of page |
Monotherapy Proof-of-Concept Studies
In their randomized, double-blind, placebo-controlled study, the Protocol 004 study team established the in-vivo tolerability, pharmacokinetic profile, and antiviral activity of raltegravir. See Sidebar.
| Naïve-patient studies | Top of page |
Protocol 004
In 2006, Markowitz et al published the results of Part II of Protocol 004, a combination antiretroviral therapy trial enrolling 201 treatment-naïve HIV-1–positive participants (including 30 from the raltegravir monotherapy trial [Part I]). In conjunction with tenofovir and lamivudine, all treated patients were randomized to receive 1 of 4 raltegravir doses (100, 200, 400, or 600 mg) twice daily versus efavirenz (600 mg/day). Inclusion criteria with respect to entry CD4+ T-cell count and HIV-1 RNA level were similar to that of Part I, as was the stratified randomization. (Those patients receiving placebo in Part I received efavirenz in Part II, while those treated with raltegravir in Part I retained the same drug dosage in Part II.) Characteristics were well balanced across treatment groups at baseline, with mean HIV-1 RNA levels ranging from 4.6–4.8 log10 copies/mL; mean CD4+ T-cell counts from 271–338 cells/mm3; and HIV-1 RNA levels >50,000 copies/mL in 55% of patients, and >100,000 copies/mL at baseline in 34% of patients.
Determinations of efficacy were based on a modified intention-to-treat (MITT) analysis with the primary endpoint being the proportion of patients achieving a plasma HIV-1 RNA level <400 copies/mL. Raltegravir was found to have a rapid and durable antiretroviral effect. By week 4, combination therapy with all study doses of raltegravir effected rapid and sustained reductions in plasma HIV-1 RNA levels, with at least 90% of patients reaching <400 copies/mL. At this time point, 60%–80% of patients in the raltegravir groups had suppressed their HIV-1 viral load to <50 copies/mL versus 25% of those treated with efavirenz (Figure 5). At the completion of 24 weeks (the primary time point for efficacy) and 48 weeks of therapy, differences between treatment groups diminished, with the plasma HIV-1 RNA level reduced to <50 copies/mL in up to 95% of all study subjects. These reductions in viral load were generally sustained through week 48 (Figure 5). The average increases in CD4+ T-cell counts were comparable across treatment groups at weeks 24 and 48. Reflecting the potency of raltegravir-based therapy, patients receiving raltegravir at any dose achieved HIV-1 RNA levels of <50 copies/mL statistically earlier than patients receiving efavirenz; the full clinical significance of this earlier virological suppression remains to be determined.
The majority of adverse events in Protocol 004 were graded mild (~85%) to moderate. Drug-related clinical adverse events were less frequent with raltegravir (48%) than efavirenz (71%). The most frequent raltegravir-related adverse events including nausea, dizziness, and headache. The incidence of serious adverse events was similar in patients receiving the raltegravir and efavirenz combination regimens (5% and 6% respectively), and the incidence of adverse events was not related to raltegravir dose. Not surprisingly, neuropsychiatric symptoms were less common with raltegravir than with efavirenz at weeks 8 and 48: 8% versus 21% and 13% versus 29%, respectively.
None of the serious adverse events in this study were considered to be drug related or led to treatment discontinuation. Additionally, grade-3 and -4 laboratory abnormalities were uncommon in this study. In patients receiving raltegravir, these included decreased absolute neutrophil count, transaminitis, or increased pancreatic enzymes. In addition, raltegravir was found to have a neutral effect on serum lipids: at week 48, the mean change from baseline in total cholesterol for raltegravir was –2.3 mg/dl versus +20.7 for efavirenz (P<0.001). Low-density lipoproteins and triglycerides were also relatively unchanged from baseline in the raltegravir groups but were increased in the efavirenz group.42
Figure 5. Efficacy differences of raltegravir versus efavirenz from Protocol 004 through week 48.
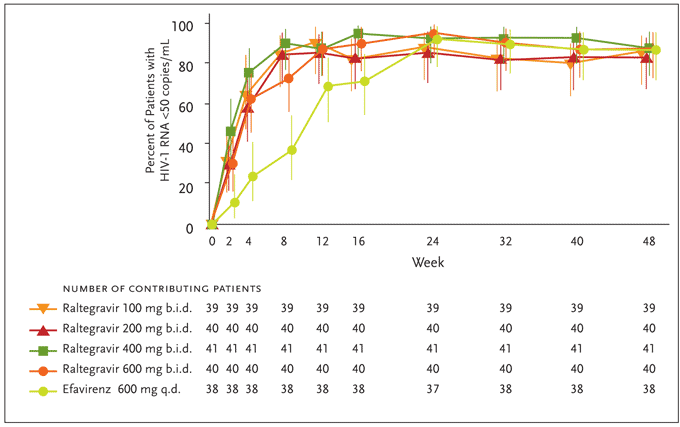
Differences versus efavirenz at Week 4 and 8 are statistically significant (P<.05).
From Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naïve patients with HIV-1 infection. J Acquir Immune Defic Syndr 2007;46(2):125-133. Reprinted with permission from Lippincott Williams & Wilkins; © 2007.
| Decay hypotheses | Top of page |
Decay Hypotheses
An intriguing aspect of the raltegravir-naïve 004 study was the documented accelerated HIV-1 RNA decay to <50 copies/mL in raltegravir-treated groups. See Sidebar Two.
| Resistance to Integrase Inhibitors | Top of page |
Virologic failures in clinical studies provide valuable information on raltegravir resistance. In the BENCHMRK studies, 41 patients in the raltegravir arm were deemed treatment failures. Mutations were described in 32 of these patients: N155H, Q148K/R/H, and infrequently Y143R/C.34,35 Data presented in abstract from the Merck Protocol 005 Study team are consistent with these findings. In their analysis of 35 patients with integrase mutations during virologic failure on raltegravir+OBR, two genetic pathways of mutations in the HIV-1 integrase gene were noted: N155H or Q148K/R/H. Both pathways were associated with raltegravir resistance, with the Q148 pathway of mutations resulting in measurably larger reductions in susceptibility (25-fold versus 10-fold for N155). The acquisition of N155 or Q148 mutations were found to result in cross-resistance to structurally diverse integrase inhibitors and the acquisition of additional mutations resulted in high-level resistance both in vitro and in vivo.43 Of note, these mutations point directly to the catalytic site of HIV-1 integrase.20 The cross-resistance exhibited by HIV-1 variants with N155 or Q148 mutations is therefore consistent with the supposition that integrase inhibition takes place by affecting binding of the common pharmacophore within the active catalytic site of HIV-1 integrase. Factors influencing selection of divergent pathways leading to resistance and their full clinical implications remain unclear.43
During in-vitro passage of wild-type HIV-1 in the presence of elvitegravir, 2 patterns of primary integrase resistance—T66I and E92Q—were found to be the most commonly selected. The E92Q mutation had the greatest effect on elvitegravir susceptibility, reducing it 33-fold, while the T66I mutation reduced susceptibility 15-fold. These assays reveal a high level of cross-resistance between elvitegravir and raltegravir, with the E92Q and T66I mutations reducing raltegravir susceptibility by 6.0-fold and 1.4-fold, respectively. Furthermore, these primary resistance mutations were often accompanied by secondary mutations in integrase. Specifically, H51Y, S147G, and E157Q were found to accompany the E92Q mutation, while F121Y (a mutation also associated with reduced raltegravir susceptibility), S153Y, and R263K accompanied the primary T66I mutation. These secondary mutations further reduced elvitegravir susceptibility.44,45
Much of the current information on elvitegravir resistance in vivo derives from an analysis of the integrase genotypes of viral isolates from protocol-defined virologic failures in the previously described phase-II, randomized, dose-finding study of elvitegravir (Gilead study 0105) in patients with heavy treatment experience. Integrase genotyping was performed on 28 of 30 patients with virologic failure in the ritonavir-boosted, elvitegravir 125-mg dosing arm by week 24. The most common integrase mutations developing in those patients were E92Q, E138K, Q148R/K/H, and N155H—each of which was observed in 39% of virologic failures; S147G (observed in 32%) and T66I/A/K (observed in 18%) complete the list of most commonly noted mutations.46 Despite their diverse structures, phenotypic analysis of HIV-1 from these patients also provided evidence for cross-resistance between the first-generation integrase inhibitors. Virus derived from virologic-failure patient samples demonstrated a mean elvitegravir fold change (FC) of greater than 151 (range 1.02–301) relative to the NL4-3 reference strain. These same samples demonstrated a raltegravir FC greater than 28-fold (range 0.78–256), consistent with reduced susceptibility across the first-generation integrase inhibitor class.46
| FDA Approval and Clinical Use | Top of page |
| Indications and Usage | Top of page |
On October 16, 2007, the FDA announced the approval of raltegravir for the treatment of HIV-1 infection as part of combination antiretroviral therapy in treatment-experienced patients with evidence of ongoing replication of HIV-1 strains resistant to multiple antiretroviral agents.47 For the treatment of patients with HIV-1 infection, the dosage of raltegravir is 400 mg administered orally, twice daily, with or without food. At present, raltegravir is the only drug in the integrase-inhibitor class approved for clinical use.
| Drug–Drug Interactions | Top of page |
In-vivo and in-vitro studies demonstrate that raltegravir is mainly eliminated via a uridine diphosphate glucuronosyltransferase 1 family, polypeptide A1 (UGT1A1)-mediated hepatic glucuronidation metabolic pathway.48 Glucuronosyltransferase inhibitors, as well as inducers of the enzyme, have the mechanistic potential to increase or decrease raltegravir concentrations, respectively. The elimination of raltegravir via the UGT1A1 metabolic pathway suggests caution be used in the coadministration of raltegravir with strong inducers of this pathway, such as rifampin, which could theoretically reduce raltegravir concentrations. The impact on UGT1A1 of other strong inducers of drug-metabolizing enzymes, such as phenytoin and phenobarbital, is unknown. Other less-strong inducers (eg, efavirenz, nevirapine, rifabutin, St. John’s wort) may be used with the recommended dose of raltegravir. Finally, unlike the PIs, raltegravir has no apparent effect on the cytochrome P450 3A4 (CYP3A4) system, and therefore has a low propensity to alter the pharmacokinetics of agents metabolized by CYP3A4.48 Additionally, raltegravir is not an inhibitor of UGT1A1, UDP glucuronosyltransferase 2 family, polypeptide B7 (UGT2B7), or P-glycoprotein-mediated transport.49
Much of the available data on drug–drug interactions with raltegravir is derived from pharmacokinetic studies presented in abstract at the 2006 Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). In a 14-day placebo-controlled study of 12 subjects receiving 400 mg raltegravir alone or in combination with 600 mg of efavirenz, the authors report a 21% reduction in C12hr and 36% reduction in both Cmax and AUC of raltegravir in the presence of the NNRTI. No significant decrease in Tmax or t1/2 was reported despite these differences.50 Similarly, the combination of 400 mg of raltegravir+100 mg ritonavir dosed twice daily had no significant impact on raltegravir pharmacokinetic parameters when compared to raltegravir administered alone.50 In healthy subjects, administration of the combination of 400 mg of raltegravir twice daily with standard-dose tenofovir for 4 days resulted in modest increases in raltegravir AUC (49%) and Cmax (64%). Cmin was unchanged. Conversely, the AUC was decreased by 10% and Cmin by 13% for tenofovir.51 These data do not suggest the need for dose adjustment with this combination. Additionally, the short-term addition of 400 mg raltegravir twice daily to steady-state tipranavir/ritonavir combination dose led to a 24% decrease in the tipranavir/ritonavir AUC. The Cmax decreased by 18%.52 Currently available information on the clinical occurrence of drug–drug interactions with raltegravir can be found in Table 1.
| Table 1. Reported Effects of Rifampin and HIV ARVs on the Pharmacokinetics of Raltegravir | |||||
| Ratio (90% CI) of Raltegravir Pharmacokinetic Parameters with/without Coadministered Drug; No Effect = 1.00 |
|||||
| Coadministered Drug Dose/Schedule | Raltegravir Dose/Schedule | n | Cmax | AUC | Cmin |
| Atazanavir 400 mg daily | 100 mg single dose | 10 | 1.53 (1.11, 2.12) | 1.72, 2.02) | 1.95 (1.30, 2.92) |
| Atazanavir 300 mg+ritonavir 100 mg daily | 400 mg single dose | 10 | 1.24 (0.87, 1.77) | 1.41 (1.12, 1.78) | 1.77 (1.39, 2.25) |
| Efavirenz 600 mg daily | 400 mg single dose | 9 | 0.64 (0.41, 0.98) | 0.64 (0.52, 0.80) | 0.79 (0.49, 1.28) |
| Rifampin 600 mg daily | 400 mg single dose | 9 | 0.62 (0.37, 1.04) | 0.60 (0.39, 0.91) | 0.39 (0.30, 0.51) |
| Ritonavir 100 mg twice daily | 400 mg single dose | 10 | 0.76 (0.55, 1.04) | 0.84 (0.70, 1.01) | 0.99 (0.70, 1.40) |
| Tenofovir 300 mg daily | 400 mg single dose | 9 | 1.64 (1.16, 2.32) | 1.49 (1.15, 1.94) | 1.03 (0.73, 1.45) |
| Tipranavir 500 mg+ritonavir 200 mg twice daily | 400 mg single dose | 15 (14 for Cmin) | 0.82 (0.46, 1.46) | 0.76 (0.49, 1.19) | 0.45 (0.31, 0.66) |
From: Isentress (raltegravir) [package insert]. Reprinted with permission from Merck & Co., Inc.49 |
|||||
| Adverse Reactions | Top of page |
The BENCHMRK studies provide the most complete information available thus far with regards to adverse events associated with the use of raltegravir. The most common adverse reactions reported in subjects in either the raltegravir or the placebo treatment group, regardless of causality, were nausea, headache, diarrhea, and pyrexia. While creatinine kinase elevations, myopathy, and rhabdomyolysis were observed in subjects receiving raltegravir, the true relationship of raltegravir to these events is currently unknown.34,35 As a result, the manufacturer recommends that raltegravir be used with caution in patients receiving concomitant medications that may place them at increased risk for these events.49 It should also be noted that although an early imbalance in the diagnosis of malignancies had been seen in the raltegravir-treated patients, this imbalance resolved with further follow-up. Post-approval surveillance will be needed to provide more definitive data on the rate, scope, and severity of integrase-inhibitor–related adverse events.
| Use in Special Populations | Top of page |
Although hepatic glucuronidation appears to be the major clearance mechanism of raltegravir in humans, clinical trials have revealed no important pharmacokinetic differences between healthy individuals and those with moderate hepatic impairment. At present, no dose adjustment appears necessary for those with mild to moderate disease. However, no recommendation can be made for the use of raltegravir in patients with severe hepatic impairment. No clinically important pharmacokinetic differences between those with severe renal impairment and healthy subjects have been reported when administered raltegravir. However, it should be noted that the extent to which raltegravir may be dialyzable is unknown.49
Raltegravir is currently classified as a category-C drug, and should therefore be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. There are no adequate clinical or pharmacokinetic studies of raltegravir in pregnant women. A similar situation exists for the pediatric patient, for whom no data from clinical trials exist.49
| Conclusions | Top of page |
As a new class of drug targeting the third essential enzyme for HIV replication (along with reverse transcriptase and protease), the integrase inhibitors are a welcome addition to the treatment armamentarium for HIV/AIDS in treatment-experienced patients failing available antiretroviral regimens.
Clinically relevant interactions with other available antiretroviral agents and long-term adverse effects and tolerability will have an impact on the future clinical value of the integrase inhibitors. Definition of the genetic barriers to integrase-inhibitor resistance, determinants of choice in the divergent pathways to resistance, and questions regarding cross-resistance across the class will need to be addressed. The integrase inhibitors should also be studied further as potential components in first-line HAART regimens based on available experimental data with raltegravir in combination with NRTIs. This would be particularly true for those in whom PI- or NNRTI-based therapy may be less than optimal. Finally, further safety, pharmacokinetic, and tolerability studies of raltegravir in special populations are warranted.
When considered as a whole, the promising efficacy and tolerability profile of the integrase inhibitors, absence of cross-resistance with other antiretroviral classes, and demonstrated synergism of the integrase inhibitors in combination with approved antiretroviral agents place them in a position to become important components of effective combination antiretroviral regimens in individuals living with HIV/AIDS.
| References | Top of page |
