A great deal of research conducted over the past ten years has established potent antiretroviral therapy as a tried and true way of drastically reducing HIV replication. Less is known about strategies to enhance T-cell production to preserve or restore immune function in HIV-infected patients. This is not to say that immune-based research and evaluation is not being conducted. Work completed at the Gladstone Institute of Virology and Immunology—not to mention the numerous other immunology labs throughout the world—is a testament to this. As the Gladstone Institute’s Dr. Laura Napolitano affirmed at the January PRN meeting, much progress has been made. Not only do we better understand the mechanisms by which CD4+ cells are lost in HIV-infected individuals and then gained in response to antiretroviral therapy, research in this regard has given rise to a number of potential pharmacologic strategies that continue to be explored in studies.
| I. The Rise and Fall of CD4+ Cells | Top of page |
Dubbed “the seat of courage” by the Greek physician Galen in the second century ad and “the abode of the soul” by other ancients, the thymus has long been an organ of great scientific interest. It was not until the 1950s that the thymus was classified as a lymphoid organ. With the explosion of immunology as a distinct biological field of study in the 1960s and 1970s, more detail regarding the function of the thymus and its relationship to the rest of the immune system rapidly emerged.
Peaking in relative size by the time of a baby’s first birthday, the thymus is an organ with a distinct cellular organization. It lies in the upper part of the mediastinum behind the sternum, extending upwards into the root of the neck. The thymus is divided into two or more segments—called lobes—composed of grape-like clusters demarcated by cortical and medullary regions. From the end of the first trimester of gestation through adolescence, the thymus clearly functions as an organ of de novo T-cell production. At puberty, however, things change quite dramatically. While the organ maintains its size and “wet” weight, the healthy-looking thymic tissue of the younger organ is gradually lost and replaced by deposits of fat. This process of “involution” is continuous, and beyond the age of 40 to 50 functioning thymic tissue is hard to find.
The process of T-cell production begins in the bone marrow, where progenitor cells are released and then migrate to the thymus for expansion and differentiation (thymopoiesis). In the thymus, maturing T-cells must undergo two selective processes. First is a positive selection to retain only those cells with T-cell receptors (TCRs) that can recognize “self” HLA molecules. This is followed by a negative selection to remove those cells with unwanted, “anti-self” TCRs. In this way, the only T-cells to survive—approximately 1% of all T-cells that enter the thymus for maturation each day—are those that can effectively engage in an immune response later. Those cells move out into the peripheral circulation as naive (CD45RA+CD62L+) CD4+ and CD8+ T-cells. Within compartmentalized lymphoid organs and upon contact with antigen in the context of appropriate antigen-presenting cells, naive T-cells then mature into CD45RO+ memory T-cells endowed with a complete array of effector functions
| Growth Hormone (GH) | Top of page |
While IL-2 appears to contribute to an increase in naive CD4+ cell populations through survival of existing cells in the periphery, research indicates that GH may increase the number of new T-cells much earlier in the production process. “We’re conducting studies in our laboratory to better understand how GH acts as an immune booster in the human body,” Dr. Napolitano explained. “Limited data suggest that it works very early in T-cell production by stimulating T-cell progenitors in bone marrow. It also may facilitate the engraftment and survival of these progenitors in the thymus.”
A number of rodent studies have been completed. GH may act directly upon immune tissues or its effects may be mediated indirectly through insulin-like growth factor-1 (igf-1). Rodents deficient in GH exhibit stress-related thymic hypoplasia that improves with GH replacement (Berczi, 1991; Murphy, 1992; Dorshkind, 2000). In older rodents, the administration of GH or igf-1 reverses age-related declines in thymopoiesis and accelerates immune reconstitution in immunodeficient animals (Kelley, 1986; Knyszynski, 1992; Bar-Dayan, 1994; Montecino-Rodriguez, 1998). What’s more, GH or igf-1 therapy accelerates immune reconstitution in immunodeficient animals (Beschorner, 1991; Murphy, 1992; Woo, 1999; Montecino-Rodriguez, 1998; Tian, 1998).
Based on the results of these rodent studies, Dr. Napolitano and her colleagues hypothesized that treatment with recombinant human growth hormone (rhGH) might reverse thymic involution and stimulate production of naive CD4+ cells in HIV-infected patients.
In a clinical trial published in a 2002 issue of AIDS, Dr. Napolitano’s team treated five HIV-infected adults with rhGH (Serostim) 3 mg/day for six to 12 months (Napolitano, 2002; 2002a). The dose was reduced to 1.5 mg per day after the first six months of therapy, except in one subject, in whom rhGH dose reduction occurred at month 2 as a result of persistent arthralgias. Immunological analyses were performed before rhGH treatment and repeated at three-month intervals after rhGH initiation. Thymic mass was analyzed using computed tomography with quantitative density and volume analysis. Analysis of circulating lymphocytes, including naive and memory T-cell subsets, was also performed using multiparameter flow cytometry.
There was a marked increase in thymic tissue in all subjects after six months of rhGH therapy (see Figure 3). At baseline, the five patients had thymic atrophy as evidenced by the near-complete replacement of the thymus by fat on ct scan (mean thymic index of one). Repeat analysis after six months of treatment revealed a prominent increase in dense thymus tissue in all of the rhGH recipients (mean thymic index of four, P = 0.0002 compared with baseline). Quantitative density and volume measurements at six months demonstrated a mean increase in thymic density of 86 Hounsfield Units (HU) (P = 0.0008), and a mean increase in thymic volume of 88% (P = 0.06). To determine whether the reversal of thymic atrophy was caused by a generalized lipolytic effect of rhGH, quantitative density analysis was performed on the axillary adipose tissue of each patient. No increase in axillary adipose density was detected (mean change of +3 HU at six months and -9 HU at one year).
Figure 3. Growth Hormone Therapy Is Associated with Increased Thymic Density and Volume
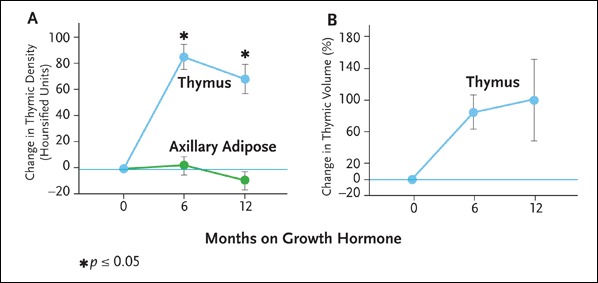
In a clinical trial reported in 2002, Dr. Laura Napolitano and her colleagues treated five HIV-infected adults with recombinant human growth hormone (rhGH) 3 mg/day for six to 12 months. The dose was reduced to 1.5 mg per day after the first six months of therapy, except in one subject, in whom rhGH dose reduction occurred at month 2 as a result of persistent arthralgias. Thymic mass was analyzed using computed tomography with quantitative density and volume analysis. Analysis of circulating lymphocytes, including naive and memory T-cell subsets, was also performed using multiparameter flow cytometry.
As is illustrated here, there was a marked increase in thymic tissue in all subjects after six months of rrhGH therapy. Quantitative density and volume measurements at six months demonstrated a mean increase in thymic density of 86 Hounsfield Units (HU), and a mean increase in thymic volume of 88%.
Source: Napolitano, 2002.
Treatment with rhGH was also associated with an increase in the percentage and absolute number of naive CD4+ cells. When compared with baseline values, the mean absolute gain in the naive CD4+ cell percentage was 6% at six months, 10% at nine months, and 12% at 12 months; the six, nine, and 12-month increases were statistically significant. Naive CD8+ cells did not increase with rhGH treatment, and no significant changes in thymic index, naive CD4+ cell percentages or naive CD8+ cell percentages were seen in historical control subjects who were maintained on effective antiretroviral therapy for a similar period of time.
Dr. Napolitano’s team followed two study participants to evaluate the effects of discontinuing rhGH on thymic function. Repeat thymus ct scans, three to twelve months after rhGH discontinuation, revealed a decrease to baseline thymic density in both individuals. However, gains in naive CD4+ cells remained stable.
A handful of studies reported over the past two years have supported the findings of Dr. Napolitano’s group. “It goes without saying that these findings do not support the general use of rhGH with the intent of reversing immune deficiency,” Dr. Napolitano said. Certain limitations of this study, including the small number of treated subjects and the lack of a randomized control arm, require that these data be interpreted with caution. There are also several significant adverse effects of growth hormone therapy that must be taken into consideration. Fortunately, additional studies are under way to evaluate further the role of rhGH as an immune-based therapy.
| References | Top of page |
