The etiology and pathogenesis of antiretroviral therapy-associated morphologic complications—most notably loss of subcutaneous fat and truncal obesity—remain something of a mystery. However, research continues to move forward. To bring PRN members up to date on the various work that is being done to better understand and manage the fat redistribution that is synonymous with HIV-associated with lipoatrophy, Dr. Donald Kotler took the podium at the November 2004 PRN meeting to review some of the newest, most important data that will likely guide clinical research in this arena in the months and years to come.
| I. Lipoatrophy | Top of page |
“Perhaps the biggest advance we’ve seen, in trying to better understand lipoatrophy, is the supplementing of in vitro data with actual prospective studies enrolling patients beginning antiretroviral therapy,” Dr. Kotler said. One study that has been referenced heavily is ACTG 384. A substudy of ACTG 384 (A5005S) was designed to monitor changes in limb fat—adipose tissue in the arms and legs—and trunk fat using dual-energy x-ray absorptiometry (DEXA) scanning in association with starting various antiretroviral combinations for the first time. ACTG 384 was the first clinical trial to prospectively follow HIV-positive patients who had not taken other antiretrovirals in the past. In turn, the ACTG investigators were able to avoid the usual limitations associated with analysis of data from retrospective and cross-sectional cohort studies, as well as clinical trials involving antiretroviral-experienced patients.
ACTG 384 was a complex study. It contained six study groups with two randomizations. The first randomization involved an open-label assignment to receive stavudine (Zerit) plus didanosine (Videx) or zidovudine (Retrovir) plus lamivudine (Epivir). The second randomization, which was blinded, assigned patients to receive nelfinavir (Viracept), efavirenz (Sustiva), or nelfinavir and efavirenz combined. Of the 980 patients enrolled into ACTG 384, 89 patients in the zidovudine/lamivudine group underwent DEXA scanning as a component of A5005S; 87 patients in the stavudine/didanosine group underwent DEXA scanning. The preliminary data from this study, reported at the 4th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV by Dr. Michael Dube, were based on 80 weeks of follow-up (Dube, 2002).
During the first 36 weeks of therapy, there was an increase in body weight, trunk fat, and limb fat in both groups of patients. “These gains were interpreted as a return to good health,” Dr. Kotler commented. “There were a number of patients enrolled with CD4+ counts in the mid to high 200s with some weight loss. Even though they didn’t have wasting, they did see their weight return, which was pretty much universal and considered to be healthy.” It was not until 12 weeks later that differences in body composition were seen between the two groups.
After 48 weeks of treatment, there was a statistically significant decrease in limb fat in the stavudine/didanosine group, compared to the zidovudine/lamivudine group. However, it should also be pointed out that limb fat also decreased among patients receiving zidovudine and lamivudine. “Another finding that is frequently overlooked was that patients receiving nelfinavir had more lipoatrophy than those receiving efavirenz. It’s very hard to single out the exact effects of these drugs, given that they were combined with NRTIs. However, there were statistically significant differences between the nelfinavir and efavirenz groups, meaning that NRTIs probably aren’t solely to blame. There appear to be at least two effects in producing lipoatrophy.”
| II. Management of Lipoatrophy | Top of page |
When it comes to the management of lipoatrophy, there are three basic principles: avoid, switch, and treat. “Avoid” refers to the selection of antiretroviral agents, at least when planning first-line regimens, that are not believed to be associated with lipoatrophy. “Switch” refers to substituting an offending antiretroviral agent with an agent not likely to be associated with lipoatrophy. Finally, “treat” refers to the use of adjunctive therapies to manage lipoatrophy, especially when changes to an existing antiretroviral regimen isn’t possible or doesn’t yield a positive outcome.
| Avoiding Lipoatrophy | Top of page |
When it comes to selecting an antiretroviral regimen, in terms of reducing the risk of side effects like lipoatrophy, Gilead study 903—much like ACTG 384—has contributed to the school of thought that stavudine is best avoided, at least initially. Gilead 903 was a randomized, double-blind studying comparing tenofovir (Viread) to stavudine. Both drugs were combined with efavirenz and lamivudine. The study enrolled approximately 600 HIV-positive patients with a mean CD4+ count of 279 cells/mm3. Two-hundred ninety-six patients received stavudine and 296 received tenofovir. One-hundred forty-four-week follow-up data were reported last year in the Journal of the American Medical Association (Gallant, 2004).
The two groups demonstrated comparable efficacy in both the on-treatment analysis and intent-to-treat analysis. Side effect risks, however, were significantly different between the two groups. Lipoatrophy was diagnosed by investigators in 19% of those receiving stavudine, compared to 3% of those receiving tenofovir. This difference was documented using DEXA scanning, although it is important to note that DEXA scanning was not performed at baseline. In the stavudine group, there was a mean reduction in limb fat of approximately 50%. The total limb fat, after 144 weeks, was 4.4 kg in the men and 6 kg in the women receiving stavudine. In the tenofovir group, mean limb fat after 144 weeks was 8.7 kg in the men and 11 kg in the women.
“To put these data into context,” Dr. Kotler explained, “the mean limb fat measurements seen in the stavudine group are very similar to limb fat measurements seen in cohorts of patients with lipoatrophy. The mean limb fat measurements seen in the tenofovir group are similar to limb fat measurements seen in our cohort of HIV-negative healthy controls at St. Luke’s-Roosevelt Hospital.”
Dr. Kotler hinted at the possibility that the results of Gilead study 903 and ACTG 384—along with those of other similar studies and the actual experiences and observations of clinicians—are shaping attitudes toward the use of stavudine as a component of first-line therapy. “Clinicians who are starting their patients on therapy now are going to be less likely to start their patients on a regimen that contains stavudine, or didanosine, than they were several years ago,” he said. “We still have to do something for those who already have lipoatrophy and need to do something about it. But for patients beginning therapy today, it really does look like we know what to avoid. In a sense, this could mark the end of lipoatrophy, at least new cases of it.”
Careful selection of a protease inhibitor is also important in terms of avoiding morphologic side effects of antiretroviral therapy. While it has been known, for quite some time, that atazanavir (Reyataz) does not cause the same lipid abnormalities as the other currently available protease inhibitors, only recently have data emerged indicating that atazanavir may be less likely to cause changes to body fat composition as well.
BMS-034 compared outcomes of 810 antiretroviral-naive patients randomized to receive either atazanavir or efavirenz, in combination with zidovudine and lamivudine (Noor, 2004). A metabolic substudy was also conducted and included both DEXA scans, to measure truncal fat and appendicular fat, and ct scans, to measure SAT and visceral adipose tissue (VAT). Two-hundred eleven patients were included in the metabolic study, which compared data collected at baseline with data collected at 48 weeks.
In short, patterns of fat gain were similar in the atazanavir and the efavirenz groups. Moderate increase in truncal fat and appendicular fat were seen in both groups, with no significant differences between the two. Similarly, moderate increases in SAT and VAT were seen in both groups, again with no significant differences between the two. “With these data,” Dr. Kotler commented, “it also looks like the promoting effect of lipoatrophy from protease inhibitors may be avoidable.”
| Treating Lipoatrophy | Top of page |
In vitro studies have demonstrated that thiazolidinediones—typically used in the management of diabetes—stimulate PPARg and increase adipogenesis, meaning that they are potentially useful as a treatment to reverse lipoatrophy. Clinical trials, involving diabetic patients without HIV infection, have demonstrated that their use are associated with increased SAT and decreased VAT.
There have been a handful of studies evaluating either rosiglitazone (Avandia) or pioglitazone (Actos) as potential treatments for lipoatrophy—and underlying metabolic complications—in HIV-infected patients. Unfortunately, the results of these studies have yielded conflicting results. Dr. Kotler briefly reviewed the results of five studies. Three studies—two open-label evaluations and one randomized, placebo-controlled trial—demonstrated statistically significant increases in SAT (Calmy, 2001; Gelato, 2002; Hadigan, 2004). One of these three studies also demonstrated a statistically significant decrease in VAT (Gelato, 2002). Neither of the other two studies demonstrated a significant increase in SAT or a decrease in VAT (Sutinen, 2002; Carr, 2004).
As was reviewed in the December 2004 issue of The PRN Notebook, and was discussed in great detail in a panel PRN presentation held in February 2005, plastic surgery and other restorative modalities are gaining momentum among HIV-infected patients who have grown frustrated with the stigma and poor self-image that accompanies lipoatrophy. “Removal of buffalo humps through plastic surgery is one option,” Dr. Kotler said, “but as many as 50% of patients who undergo this procedure see a recurrence.” As for correction of facial and buttock lipoatrophy, Dr. Kotler explained that approximately 20 options are available through the international market, mostly in Europe. But permanent and non-permanent fillers are available, although Sculptra—a non-permanent filler—is the only product to be approved by the U.S. Food and Drug Administration specifically for the restoration of facial fat loss. “Bovine collagen for lipoatrophy is the most widely used method in the United States,” he said. “It is associated with few adverse events, but is reabsorbed over three to six months. As for autologous fat transfer, this has proved difficult in lipoatrophic patients. If fat is harvested from dorsocervical fat pads”—some surgeons have done this, especially when there is a lack of SAT that can be harvested from other parts of the body—“it appears to endure longer than SAT as a facial implant, but there is the possibility that it will continue to grow.”
| III. Truncal Obesity | Top of page |
Attempts to come up with a case definition for lipodystrophy have been fraught with contradictions. For example, some data have led experts to question the assumption that the two main morphologic complications—lipoatrophy and truncal obesity—being seen in HIV-positive patients can actually be categorized as being of the same syndrome. A prime example of this can be found in data from the Fat Redistribution and Metabolic Change (FRAM) study, a multi-site, cross-sectional cohort (Gripshover, 2003).
FRAM randomly selected 1200 HIV-positive patients through various HIV research sites in the United States and 300 HIV-negative controls (randomly selected from the Coronary Artery Disease Risk Development in Young Adults [cardia] study, sponsored by The National Heart, Lung, and Blood Institute). This allowed for a number of key comparisons using two distinct patient populations. FRAM was not conducted with any preconceived notions as to what the syndrome is. And for factors to be considered a part of an HIV-specific syndrome, there needed to be statistically significant differences between the HIV-positive patients and the HIV-negative controls. There also needed to be statistically significant positive associations between two factors for them to be included in the same syndrome.
With respect to lipoatrophy, HIV-positive men in the FRAM study were significantly more likely to self-report peripheral fat loss—in the cheeks, face, arms, legs, and buttocks—whereas HIV-negative controls were more likely to report gains in peripheral fat. Using MRI, the FRAM investigators found that HIV-positive men, regardless of whether or not they self-reported lipoatrophy, had significantly less SAT than HIV-negative controls. And among the HIV-positive men, MRI evidence of lipoatrophy was more pronounced in those who did self-report lipoatrophy than those who did not. Peripheral fat in the legs suffered the most profound loss, followed in decreasing order by peripheral fat in the arms, lower torso, upper torso, and back.
Perhaps the most striking and unexpected finding was the comparison of VAT content between the two groups. The HIV-positive subjects were no more likely than the HIV-negative controls to self-report increases in abdominal fat. Turning to the MRI data, the FRAM investigators determined that VAT was somewhat lower in the HIV-positive patients when compared to the HIV-negative controls—a finding that was statistically significant. In other words, Dr. Kotler explained, there was no linkage between fat lipoatrophy and fat accumulation. HIV-positive patients with lipoatrophy weren’t any more likely to experience changes in VAT than HIV-positive patients without lipoatrophy.
At odds with these preliminary study results are data from another study reported at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, held in November 2004 (Saag, 2004). This study involved a group of male patients who had waist circumferences greater than 88.2 cm and waist-to-hip ratios greater than 95. A number of characteristics were examined and results from 207 HIV-infected men were compared with results from a control group of 144 HIV-negative men. VAT and SAT were measured by ct scans in the HIV-infected men and by MRI in the HIV-negative men.
The HIV-infected men had a significantly greater amount of VAT than did HIV-negative men with the same waist circumference. Through multiple logistic regression analyses, the investigators determined that BMI and age had minimal impact on differences between the HIV-infected men and the HIV-negative men. While these data suggest that HIV infection is associated with the accumulation of VAT, weaknesses of this study are apparent. For starters, it is not an epidemiologic study; participants were recruited on the basis of existing accumulation of central adipose tissue. Moreover, the data do not allow for a clear differentiation between the two factors that may account for the observed VAT increase: an actual higher absolute quantity of VAT or a lower absolute SAT due to lipoatrophy. “I’m not sure what’s going on,” Dr. Kotler commented. “We do see an increase in VAT among HIV-infected patients, but we also see an increase in VAT among HIV-negative patients as well. So it’s not clear what we’re seeing: a significant difference in VAT among HIV-positive patients or, more likely, significant decreases in SAT that are skewing VAT comparisons between HIV-positive patients and HIV-negative people.”
| IV. Management of Truncal Obesity | Top of page |
“Regardless of what’s causing visceral fat accumulation, whether it’s seen more or less in HIV or which antiretroviral agents it is associated with, it’s still a problem that needs to be dealt with,” Dr. Kotler said. “In some ways, we need to get over the whole argument about what it is and focus on finding and using an effective treatment. And the only treatment that groups have really looked at so far is growth hormone.”
Some of the more recent data involving recombinant human growth hormone (rhGH) for truncal obesity was reported at the 11th Conference on Retroviruses and Opportunistic Infections, held in San Francisco in February 2004 (Kotler, 2004). This prospective, multicenter, randomized, dose-finding extension study evaluated the efficacy and safety of rhGH maintenance therapy, 1 mg or 2 mg daily, to sustain reductions of VAT and cholesterol concentrations achieved using higher-dose rhGH. In the original study—dubbed the Serostim for the Treatment of Adipose Redistribution Syndrome (STARS) trial—rhGH doses of 4 mg a day for 12 weeks significantly reduced trunk fat, VAT, total cholesterol, and non-HDL cholesterol, compared to placebo (see Figure 2).
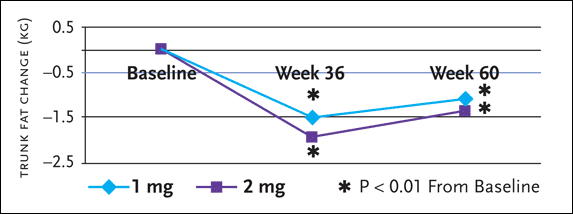
Figure 2.rhGH Maintenance Therapy for Truncal Obesity
Preliminary results from a prospective, multicenter, randomized, dose finding extension study evaluation the efficacy and safety of recombinant human growth hormone (rhGH) maintenance therapy, 1 mg or 2 mg daily, to sustain reductions of VTA achieved using higher-dose rhGH, in the original study dubbed the Serostim for the Treatment of Adipose Redistribution syndrome (STARS) trial-rhGH doses of 4 mg a day for 12 weeks significantly reduced trunk fat, VAT, total cholesterol, and non-HDL cholesterol, compared to placebo.
Significant reductions from the start of the STARS trial to week 60 were found in both the 1 mg and 2 mg maintenance groups for trunk fat (-1.1 and -1.4 kg from 9.5 and 9.8 respectively). There were no between group differences in any parameters from baseline to weeks 36 or 60 among patients who received growth hormone 1 mg or 2 mg maintenance therapy.
SourceKotler, 2004
Subjects in the reported extension study included 142 HIV-positive patients with excess VAT, without glucose intolerance, who initially had been randomized to rhGH 4 mg/day, alternate-day rhGH therapy, or to placebo in the STARS trial for 24 weeks, and then were re-randomized to rhGH 4 mg/day or alternate days for the first 12 weeks (weeks 24 to 36 from initiation of the STARS trial). Subsequently, 127 were re-randomized to receive 24 weeks of maintenance therapy (1 mg or 2 mg daily) for an additional 24 weeks (36 to 60 weeks from STARS trial baseline).
Among clinical endpoints assessed (at baseline, weeks 12, 24, 36, and 60) were trunk fat (measured using DEXA scans), total cholesterol, non-HDL cholesterol, and glucose tolerance testing.
Significant reductions from the start of the STARS trial to week 60 were found in both the 1 mg and 2 mg maintenance groups for trunk fat (–1.1 and –1.4 kg from 9.5 and 9.8 kg respectively), non-HDL cholesterol (–21.2 and –23.8 from 175.6 and 172.1 mg/dL respectively), and total cholesterol (–16.9 and –18.5 from baselines of 213.0 and 209.2 mg/dL respectively). There were no between-group differences in any parameters from baseline to weeks 36 or 60 among patients who received growth hormone 1 mg or 2 mg maintenance therapy, nor were there any significant differences in the incidence of common adverse events, with the exception of arthralgia—12.5% on 2 mg and 5.7% on 1 mg—during the 24 weeks that these therapies were administered.
“One of the main side effects we worry about with Serostim, especially when using superphysiologic doses of the drug, is insulin resistance,” Dr. Kotler explained. “We saw an increase out to 36 weeks, but the insulin levels came back down after 60 weeks, but they didn’t quite come back down to normal. If we’re talking about trying to reduce cardiovascular risk by reducing cholesterol, we still need to be concerned about increased insulin levels. Fortunately, there will be at least one study evaluating growth hormone in combination with rosiglitazone, aimed at patients with VAT accumulation and insulin resistance.”
The study Dr. Kotler referred to is being funded by the National Insitutes of Health and will be conducted at St. Luke’s-Roosevelt Hospital and on both campuses (Cornell and Columbia) of New York Presbyterian Hospital. For the first 12 weeks of the study, patients will be randomized evenly to receive either rhGH (2 mg every other day) plus rosiglitazone (4 mg twice daily), rhGH plus rosiglitazone placebo, rhGH placebo plus rosiglitazone, or rhGH placebo plus rosiglitazone placebo. For weeks 13 through 24, all study participants will receive open-label rhGH plus rosiglitazone. All subjects will undergo oral glucose tolerance testing with insulin levels at screening at weeks 4, 12, and 24 and will undergo intravenous glucose tolerance tests at entry and week 12. Basal free fatty acid flux, resting energy expenditure, and energy intake will also be determined at entry and week 12. Patients will also undergo total body MRI scanning at St. Luke’s-Roosevelt at entry, week 12, and week 24 to measure VAT and SAT. DEXA scans and sodium bromide and deuterated water dilution tests will also be done at entry and week 12.
| Conclusion | Top of page |
In summary, Dr. Kotler reiterated his belief that new incidences of lipoatrophy in the United States may soon be on the decline, given the awareness among clinicians that stavudine can and should be avoided where possible. “As for the incidence of lipoatrophy in the developing world,” Dr. Kotler added, “we need to pay close attention to the medications that are recommended and the medications that actually make their way into the hands of patients in poor nations. But in developed nations, where there are numerous antiretrovirals to choose from, we’re probably on the cusp of seeing a decrease—and quite possibly the disappearance—of new cases of lipoatrophy. Of course, there will still be a residual burden of patients who have already developed lipoatrophy and will continue to need care and treatment options for it.”
As for truncal obesity, Dr. Kotler reckons that both its incidence and prevalence will continue and that it will continue to be difficult to distinguish it from the metabolic syndrome not associated with HIV, since that may be the major pathogenesis influence. “I don’t really care who wins the argument about what’s causing truncal obesity and metabolic problems,” he said. “The fact is, these problems are bad for our patients, no matter what the cause is. Fortunately, we’re beginning to see some data that may allow clinicians to select regimens that are less likely to cause these problems, which appears to be the best current solution.”
| References | Top of page |
