Endocrine abnormalities—specifically androgen deficiency—are nothing new among HIV-positive patients. Their significance came to light in the earlier days of the AIDS epidemic, particularly as a leading contributor to AIDS-related weight loss and wasting syndrome. While these complications are much less common today, thanks to the restorative benefits of antiretroviral therapy, androgen deficiency is still an issue that many HIV-positive individuals continue to grapple with. Fortunately, there have been a number of studies reported in recent years evaluating the safety and effectiveness of androgen replacement therapy in both men and women. Dr. Steven Grinspoon has played no small role in many of these studies, and thus was considered to be the ideal candidate to address the PRN membership at a recent meeting.
| I. Androgen Deficiency and Supplementation in Men | Top of page |
Hypogonadism has long been one of the most common endocrine abnormalities in HIV-positive men. Testosterone is the primary sex hormone and is secreted by the testes and the pituitary gland in response to gonadotropin stimulation.
The diagnosis of hypogonadism in men is relatively straightforward and is based on comparison with the age- and sex-adjusted normative range for serum testosterone concentrations. With respect to methodology, Dr. Grinspoon stressed that the free (bioavailable) testosterone level is the marker to keep an eye on. “Total testosterone is less accurate, given that HIV-infected patients often have increased sex hormone-binding globulin concentrations,” he explained. SHBG levels may increase the total testosterone level, but will not affect the free testosterone concentration, which is measured using equilibrium dialysis or another SHBG-independent technique.
It is also important to note that different labs use different reference ranges for free and total testosterone levels. Labs have also been known to change their normative ranges periodically, meaning that the definition of “deficiency” is far from absolute.
In one study reported in 1988, testosterone levels were evaluated in 70 HIV-infected men with asymptomatic disease (n = 19), mild symptomatic disease (n = 9), or AIDS (n = 42) (Dobs, 1988). Approximately 50% of the men with AIDS were hypogonadal. Mean serum testosterone concentrations in men with mild symptomatic disease (292 ng/dl) and AIDS (401 ng/dl) were significantly less than in men with asymptomatic disease (567 ng/dl) and HIV-negative male controls (608 ng/dl).
Manifestations of Androgen Deficiency
Dr. Grinspoon’s group has also documented gonadal deficiency in approximately 50% of HIV-infected men with AIDS-related wasting syndrome (Grinspoon, 1992). And while hypogonadism has become less common in the era of combination antiretroviral therapy, it is still documented in up to 20% of patients (Rietschel, 2000). In turn, Dr. Grinspoon stressed the importance of gonadal function testing in all patients with HIV infection, particularly in the setting of otherwise unexplainable symptoms such as fatigue, loss of energy, depression, changes in the pattern of hair growth (loss of pubic or axillary hair), less frequent need to shave, testicular atrophy, decreased libido, and gynecomastia. The suggested approach for the evaluation of HIV-infected men with symptoms consistent with hypogonadism is illustrated in Figure 1.
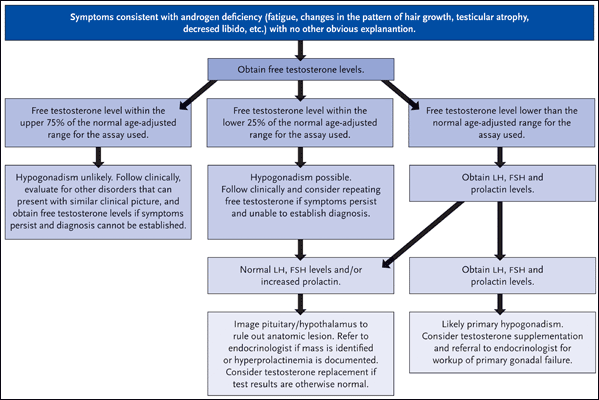
Figure 1.Evaluation of Androgen Deficiency in HIV-Infected Men
Suggested general approach for the evaluation of HIV-infected men with symptoms consistent with hypogonadism.
Source: Mylonakis, 2001. Reprinted with permission of Clinical Infectious Diseases and the Infectious Disease Society of America.
Aside from its contributions to symptoms associated with decreased quality of life, hypogonadism may also play a role in more serious complications seen in HIV-infected people, including a critical loss of lean body mass (typically seen in AIDS-related wasting syndrome). In one study published in 1996 by Dr. Grinspoon and his colleagues, there was a significant association between body cell mass, as measured by potassium isotope analysis, and serum free testosterone concentrations. Testosterone depletion has also been linked to insulin resistance, anemia, osteoporosis, dyslipidemia, and increased truncal fat.
Mechanisms of Androgen Deficiency
Testosterone deficiency in the setting of HIV has both primary and secondary causes. Primary causes, which account for approximately 25% of HIV-positive patients with hypogonadism, involve etiologies that can cause gonadal failure. Examples include opportunistic infections, gonadal malignancies, and cytokine damage to the testes. Tumor necrosis factor (TNF) and interleukin-1 (IL-1)-both of which have been shown to be upregulated in HIV infection-have been linked to decreased testicular steroidogenesis.
Secondary causes, which account for approximately 75% of HIV-positive patients with hypogonadism, act on the pituitary-or neuroendocrine-level. One example is the effect of chronic illness on the hypothalamic-pituitary-gonadal (HPG) axis. "This is similar to changes in the thyroid axis that are seen during illness," Dr. Grinspoon commented. "Fortunately, the effects of illness on the HPG access are often reversed once the illness has been managed successfully, which explains why we don't often see changes in the HPG axis in HIV-positive folks being successfully treated with antiretroviral therapy." Dr. Grinspoon also pointed out that HIV itself can lead to destruction of the pituitary gland.
Testosterone Supplementation
In one early clinical trial, a team headed by Dr. Grinspoon examined the effects of physiologic testosterone administration on body composition, exercise functional capacity, and quality of life in 51 androgen-deficient men with the AIDS-related wasting syndrome (Grinspoon, 1998). Androgen deficiency was defined as a free testosterone level less than 42 pmol/L and wasting was defined as a body weight less than 90% of ideal body weight or weight loss greater than 10% of baseline body weight. Patients were randomly assigned to receive testosterone enanthate (300 mg) or placebo intramuscularly every three weeks for six months.
Change in fat-free mass was the primary end point. Secondary clinical end points were weight, lean body mass, muscle mass, exercise functional capacity, and change in perceived quality of life. Virologic variables were assessed by CD4+ cell counts and viral load. At baseline, the median CD4+ cell count was 175 cells/mm3. The median loss of body weight was 18% and the median free testosterone level at study entry was 8.5 mg/mL.
Compared with patients who received placebo, testosterone-treated patients gained fat-free mass (–0.6 kg vs. 2.0 kg), lean body mass (0.0 kg vs. 1.9 kg), and muscle mass (–0.8 kg vs. 2.4 kg). These differences were all statistically significant. Changes in body weight, fat mass, total-body water content, and exercise functional capacity did not significantly differ between the groups. Patients who received testosterone reported benefit from the treatment: feeling better, improved quality of life, and improved appearance.
Testosterone was well tolerated in all patients. “We did not document any significant adverse events related to testosterone administration,” Dr. Grinspoon said, “most likely because we were using physiologic doses, as opposed to superphysiologic doses. In turn, we concluded that physiologic doses of testosterone in HIV-positive men with weight loss could be used to improve lean body mass, muscle mass, and quality of life.”
Dr. Grinspoon’s group has also evaluated the safety and efficacy of testosterone administration in eugonadal men—men with normal baseline free testosterone levels—experiencing AIDS-related wasting (Grinspoon, 2000). “This is a study that community activists were asking us to do,” Dr. Grinspoon explained. “It really was a different study than the one we did looking at testosterone replacement therapy in the men with hypogonadism. Here we’re talking about giving a true pharmacologic dose of testosterone, along with resistance exercise training, in patients with weight loss.”
Fifty-four men were enrolled, all of whom had free testosterone levels greater than 42 pmol/L and were either less than 90% of their ideal body weight or had self-reported weight loss of greater than 10%. Patients were randomized to one of four treatment groups: 1) testosterone plus resistance training, 2) testosterone without resistance training, 3) placebo plus resistance training, or 4) placebo without resistance training. The testosterone dose used was 200 mg, injected intramuscularly, every week. Training consisted of supervised progressive strength training and aerobic conditioning three times per week for 12 weeks.
At baseline, the median body mass index was 22.1 kg/m2 and patients, on average, reported having lost 16% of their usual body weight. Seventy-six percent of the patients were receiving some form of antiretroviral therapy and 76% had a history of a previous opportunistic infection.
Interestingly, resistance training had a significant effect on lean body mass—an increase of 2.3 kg—and muscle area, independent of testosterone administration. “[This] increase in lean body mass in response to exercise alone,” Dr. Grinspoon’s group wrote in their Annals of Internal Medicine summary of the data, “is equivalent to the effects of lower doses of testosterone and those of anabolic steroids.”
Testosterone administration also resulted in statistically significant increases in lean body mass, muscle area, and muscle strength. Lean body mass increased 4.2 kg in response to testosterone therapy without resistance training. While the study also suggested that muscle mass and strength was further increased in response to combined testosterone therapy and resistance training, “the study was not designed to compare changes in the combined treatment groups with those in the individual treatment groups.”
As for adverse effects, testosterone therapy was generally well tolerated. Levels of HDL cholesterol increased in response to training, but decreased in response to testosterone therapy. The median CD4+ cell count did not change significantly, nor did aminotransferase or prostate-specific antigen levels. Three patients developed breast tenderness or gynecomastia (one of whom was receiving placebo). No deaths or significant morbidities were reported during the 12-week study.
Also of interest are data published by Judith Rabkin, PhD, and her colleagues at the New York State Psychiatric Institute (Rabkin, 2000). Seventy-four patients with deficient or low-normal (<500 ng/dL) serum testosterone levels were enrolled in a double-blind, placebo-controlled six-week trial with 200 mg to 400 mg biweekly testosterone injections, followed by 12 weeks of open-label maintenance treatment. Major outcome measures were mood, energy, and erectile function. Body composition changes were also assessed using bioelectric impedance analysis (BIA).
Seventy men completed the trial. Response rates, defined as much or very much improved libido, were documented in 28/38 (74%) patients randomized to testosterone, and 6/32 (19%) placebo-treated patients. Of the 62 evaluable patients with fatigue at baseline, 20/34 (59%) receiving testosterone and 7/28 (25%) receiving placebo reported improved energy. Among the 26 evaluable patients with an Axis I depressive disorder at baseline, 58% of the testosterone-treated patients reported improved mood compared with 14% of placebo-treated patients. The average increase in muscle mass over 12 weeks was 1.6 kg for all men receiving testosterone injections, and 2.2 kg for the 14 men with documented wasting at baseline. Improvement on all parameters was maintained during subsequent open-label treatment for up to 18 weeks.
“The conclusions from studies evaluating testosterone therapy in HIV-infected men are apparent,” Dr. Grinspoon commented. “Testosterone replacement therapy in hypogonadal men results in improved lean mass, functional status and quality of life, without observed side effects over six months.” Replacement therapy is likely sustainable over the long-term, he said, “but it may be appropriate to retest testosterone levels if health improves.” As for pharmacologic (super-physiologic) testosterone therapy, Dr. Grinspoon commented that this also improves lean mass and strength, but is associated with decreased HDL and is not appropriate for long-term use in HIV-infected patients. “Longer term functional and survival data related to the use of testosterone in either hypogonadal or eugonadal patients are not available,” he said. “PSA and other safety indices should be monitored in all patients receiving testosterone.”
Examples of testosterone formulations available for the treatment of hypogonadism are described in Table 1.
| Table 1. Examples of Available Treatment Options for the Management of Hypogonadism Among HIV-infected Men | ||||
| Trade Name (manufacturer) | Available formulations | Usual dosage | Side effects | CD4+ Count (%) |
| Nonscrotal transdermal patch | ||||
| Androderm (Watson Laboratories) or Tostoderm TTS (ALZA Pharmaceuticals) |
Androderm: 2.5 mg or 5 mg
Testoderm TTS: 5 mg |
5 mg/day | Allergic contact dermatitis; erythema and pruritis at application site | Should not be applied over any bony prominence or on part of body that could be subject to prolonged pressure during sleep or sitting; application of small amount of 0.1% triamcinolone acetonide cream to skin under central reservoir can reduce skin irritation |
| Scrotal transdermal patch | ||||
| Testoderm (ALZA Pharmaceuticals) | 4 mg or 6 mg | 6 mg/day | Skin irritation | Difficult to ensure adequate absorption and delivery rate; maintaining adhesion can be problematic |
| Gel | ||||
| AndroGel (Unimed, Inc.) | 1% Gel | 5 to 7.5 g/day | Vasodilation, accidental exposure of sex partners | Should not be applied to genital area |
| Intramuscular injection | ||||
| (testosterone cypionate; Pharmacia & Upjohn), Delatestryl (testosterone enanthate; Bio-Technology General Co.) | Depo-Testosterone, 200 mg/mL; Delatestryl, 200 mg/mL | 200 mg every 2 weeks |
Local irritation and pain; IM injections contraindicated in event of bleeding diathesis or thrombocytopenia |
Improved compliance and higher peak testosterone levels |
| Source: Mylonakis, 2001. | ||||
| References | Top of page |
