A great deal of research conducted over the past ten years has established potent antiretroviral therapy as a tried and true way of drastically reducing HIV replication. Less is known about strategies to enhance T-cell production to preserve or restore immune function in HIV-infected patients. This is not to say that immune-based research and evaluation is not being conducted. Work completed at the Gladstone Institute of Virology and Immunology—not to mention the numerous other immunology labs throughout the world—is a testament to this. As the Gladstone Institute’s Dr. Laura Napolitano affirmed at the January PRN meeting, much progress has been made. Not only do we better understand the mechanisms by which CD4+ cells are lost in HIV-infected individuals and then gained in response to antiretroviral therapy, research in this regard has given rise to a number of potential pharmacologic strategies that continue to be explored in studies.
| I. The Rise and Fall of CD4+ Cells | Top of page |
Dubbed “the seat of courage” by the Greek physician Galen in the second century ad and “the abode of the soul” by other ancients, the thymus has long been an organ of great scientific interest. It was not until the 1950s that the thymus was classified as a lymphoid organ. With the explosion of immunology as a distinct biological field of study in the 1960s and 1970s, more detail regarding the function of the thymus and its relationship to the rest of the immune system rapidly emerged.
Peaking in relative size by the time of a baby’s first birthday, the thymus is an organ with a distinct cellular organization. It lies in the upper part of the mediastinum behind the sternum, extending upwards into the root of the neck. The thymus is divided into two or more segments—called lobes—composed of grape-like clusters demarcated by cortical and medullary regions. From the end of the first trimester of gestation through adolescence, the thymus clearly functions as an organ of de novo T-cell production. At puberty, however, things change quite dramatically. While the organ maintains its size and “wet” weight, the healthy-looking thymic tissue of the younger organ is gradually lost and replaced by deposits of fat. This process of “involution” is continuous, and beyond the age of 40 to 50 functioning thymic tissue is hard to find.
The process of T-cell production begins in the bone marrow, where progenitor cells are released and then migrate to the thymus for expansion and differentiation (thymopoiesis). In the thymus, maturing T-cells must undergo two selective processes. First is a positive selection to retain only those cells with T-cell receptors (TCRs) that can recognize “self” HLA molecules. This is followed by a negative selection to remove those cells with unwanted, “anti-self” TCRs. In this way, the only T-cells to survive—approximately 1% of all T-cells that enter the thymus for maturation each day—are those that can effectively engage in an immune response later. Those cells move out into the peripheral circulation as naive (CD45RA+CD62L+) CD4+ and CD8+ T-cells. Within compartmentalized lymphoid organs and upon contact with antigen in the context of appropriate antigen-presenting cells, naive T-cells then mature into CD45RO+ memory T-cells endowed with a complete array of effector functions
| CD4+ Cell Decline in HIV Infection | Top of page |
As was reviewed in an article summarizing a lecture by Dr. Joseph M. McCune (see “Mechanisms of T-Cell Depletion and Restoration in HIV Disease” in the September 2002 issue of The PRN Notebook)—Dr. Napolitano’s colleague at the Gladstone Institute and the University of California, San Francisco—there are essentially two distinct ways in which CD4+ cell counts become depleted in untreated HIV infection (see Figure 1).
Figure 1. CD4+ Cell Decline During HIV Infection
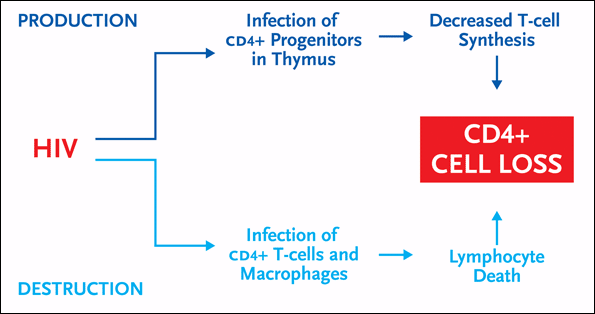
There are two distinct ways in which CD4+ cell counts become depleted in untreated HIV infection. First there is the high-turnover model, which involves the destructive effects of HIV on CD4+ cells and macrophages in the periphery (bottom half of figure). There is also the regenerative failure model, which involves the inhibitory effects of HIV on the production of new CD4+ cells in the thymus (top half of figure).
Source: Laura Napolitano, MD
First there is the high-turnover model, which involves the destructive effects of HIV on CD4+ cells and macrophages in the periphery (e.g., the peripheral lymphoid organs). There are both direct and indirect mechanisms by which this occurs. Direct destruction of infected cells is associated with viral envelope-triggered apoptosis, disruption of cell membrane integrity/syncytia formation, and accumulation of unintegrated viral DNA. There are also mechanisms that cause indirect destruction of uninfected cells. Examples include cytolysis by HIV-specific cytolytic T-cells or by natural killer cells, and T-cell activation.
There is also the regenerative failure model, which involves the inhibitory effects of HIV on the production of new CD4+ cells in the thymus. And much like the destructive effects of HIV on CD4+ cells in the periphery, there are both direct and indirect mechanisms by which HIV can impair CD4+ cell production in the thymus. Direct effects include HIV-mediated death of progenitor cells. Indirect effects include cytokine dysfunction and HIV-induced apoptosis.
“Since there are a number of potent antiretroviral therapies that are now available to decrease HIV replication in the periphery and thereby decrease the destruction caused by HIV,” Dr. Napolitano explained, “what we’re most interested in now is in finding ways to enhance CD4+ cell production so that people with HIV infection can better restore and better preserve their immune system. HIV-infected adults in particular have a big problem with T-cell production. Not only does HIV have the ability to infect and destroy the thymus, most adults don’t have much of a functioning thymus to begin with, meaning that the gland itself isn’t readily able to produce many new T-cells, even if HIV isn’t present.”
| II. Potential Enhancers of CD4+ Cell Production | Top of page |
To enhance CD4+ cell production—most notably naive CD4+ cells, which are diminished in HIV infection and can be very difficult to replace with antiretroviral therapy alone—Dr. Napolitano and her colleagues, as well as other investigators interested in immune reconstitution, have been focusing on positive regulators with potential to increase the number of naive T-cells in the body: interleukin-2 (IL-2), growth hormone (GH), and interleukin-7 (IL-7).
Just as a loss of red blood cells or platelets is naturally countered with the production of erythropoietin or thrombopoietin to stimulate the proliferation of early erythroid or megakaryocytic progenitors, HIV-induced depletion of CD4+ cells might elicit one or more factors that stimulate the production of CD4+ cells in the thymus and elsewhere. And when natural production of regulators may not be substantial enough to spark CD4+ cell production, therapeutic administration of these products may be beneficial.
| Interleukin-2 (IL-2) | Top of page |
The cytokine IL-2 is a central regulator of T-cell function (see Figure 2). It is produced by antigen-activated T-cells and induces proliferation of both CD4+ cells and CD8+ cells. It also promotes maturation and cytotoxicity activity of CD8+ cells; stimulates immune responses against viruses and intracellular organisms (the th1 response); regulates the tempo and magnitude of the immune response; and also potentiates function of antigen-presenting cells, NK cells, and B-cells. More recent data suggest that IL-2 also plays a crucial role in regulating autoimmune responses.
IL-2 effects on the thymus are still being elucidated. While IL-2 administration has been shown to increase the concentration of circulating naive CD4+ cells, even in patients with advanced HIV disease, this has been attributed primarily to the increased proliferation and decreased apoptosis of these cells in lymph nodes and the periphery.
Figure 2. IL-2 and IL-2 Receptor Complex
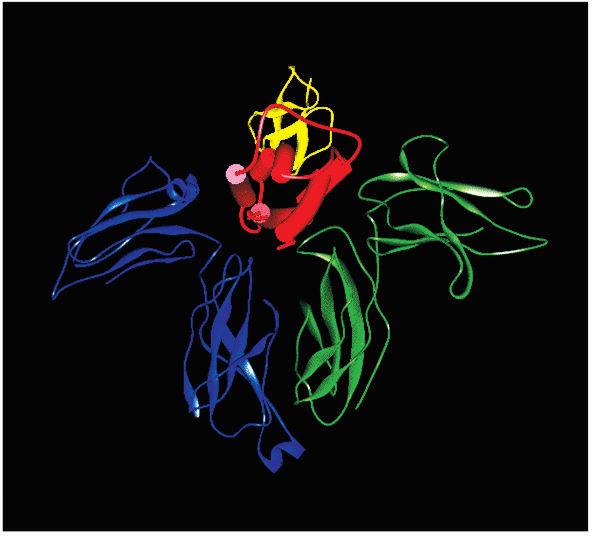
Interleukin-2 is represented schematically in red. The receptor-alpha chain is blue, the beta chain is yellow, and the gamma chain is green.
Source: Interleukin-2 is represented schematically in red. The receptor-alpha chain is blue, the beta chain is yellow, and the gamma chain is green.
Despite the fact that IL-2 is not (yet) approved as an immune-based therapy in HIV infection in the United States, its safety and efficacy—using various doses—have been evaluated in numerous clinical trials since the 1980s. The doses used by most investigators have ranged from 9 million international units (MIU) a day to 15 MIU a day, given for five days and repeated every eight weeks.
Prior to the current era of triple-drug antiretroviral therapy, IL-2 was usually evaluated in clinical trials involving patients receiving either single or dual nucleoside reverse transcriptase inhibitor (NRTI) therapy. Consistent improvement in CD4+ cell counts was documented in most patients receiving IL-2 therapy, along the lines of a 200 to 500 CD4+ cell/mm3 advantage measured seven weeks following the five-day treatment course compared with patients receiving NRTI therapy alone. Conversely, CD4+ cell counts rarely improved in patients who started IL-2 therapy with fewer than CD4+ 200 cells/mm3 at baseline, with worsening of viral load and clinical status seen in some of these patients.
In studies conducted in more recent years, IL-2 combined with more potent antiretroviral therapy has been found to generally result in CD4+ count improvements of 100 to 600 cells/mm3 over antiretroviral therapy alone when monitored seven weeks after a five-day treatment course. No significant increases in HIV viral load have been documented—there have been some reports of transient increases, as well as reports of viral load decreases in some studies—and no clear effect on latent viral reservoirs. And unlike studies exploring IL-2 in earlier years, IL-2 combined with potent antiretroviral therapy is often associated with significant CD4+ cell count increases in patients beginning IL-2 therapy with fewer than 200 CD4+ cells.
The results of recently closed Phase III studies—including the SILCAAT study (initiated by Chiron but subsequently turned over to the University of Minnesota and the National Institutes of Health) and the ESPRIT study (at the National Institutes of Health)—are very much anticipated. SILCAAT enrolled HIV-infected individuals who had less than 300 CD4+ cells/mm3, while ESPRIT enrolled individuals who had greater than 300 CD4+ cells/mm3. Both trials compare the progression to an AIDS-defining diagnosis of patients who are randomized to receiving antiretroviral therapy alone compared to those receiving antiretroviral therapy plus IL-2.
During the initial years of IL-2 research involving HIV-infected patients—most notably in studies conducted at the National Institutes of Health campus in Bethesda—IL-2 was administered by intravenous infusion. The typical dose was 18 MIU per day, administered continuously for five days, every eight weeks. Moderately severe toxicities were common, including severe flu-like symptoms, fever, bone marrow toxicity, hypotension, phlebitis, gastrointestinal toxicity, renal insufficiency, and dermatologic reactions. “Most clinicians who have experience with intravenous IL-2 know that it often provided significant benefits with respect to CD4+ cell counts,” Dr. Napolitano said. “At the same time, it had a huge toll on some of the people who were taking it.”
Dosing in more recent studies, including SILCAAT and ESPRIT, has involved 4.5 or 7.5 MIU of IL-2 administered subcutaneously twice daily for five days every four to eight weeks. “In some cases,” Dr. Napolitano added, “where we see prolonged increases in CD4+ cell counts using subcutaneous IL-2, it may be possible to extend the interval between dosing to 12 months or longer.” She also noted that while the frequency and severity of toxicities are diminished with these lower doses of subcutaneous IL-2 therapy, toxicities are still considerable.
Studies conducted in New York, under the direction of Dr. Kendall Smith, have developed an alternative approach to IL-2 therapy (see “Interleukin-2: Use in Immune Restoration and During Structured Treatment Interruption” in the March 2000 issue of the Notebook). Because the IL-2 receptor expressed by antigen-activated T-cells has a very high affinity for IL-2, Dr. Smith’s group administers much lower doses of IL-2: 2 million units a day. At this dose, Dr. Smith has demonstrated that peak plasma IL-2 concentrations are sufficient to saturate the high affinity receptors, but do not bind appreciably to the lower affinity IL-2 receptors expressed by natural killer (NK) cells. Accordingly, the systemic toxicity associated with high-dose IL-2 administration can be circumvented, and a low therapeutic concentration of IL-2 can be achieved in total-body extracellular space.
IL-2 can be prescribed in the United States, but is approved by the U.S. Food and Drug Administration only for the treatment of renal cell cancer. In Europe, it is approved for the treatment of HIV in patients with fewer than 200 CD4+ cells/mm3. “Usage in HIV disease is still considered experimental in the U.S.,” Dr. Napolitano said. “While it can be prescribed for HIV, this is considered an off-label use of the drug. If off-label use is pursued, patients should be monitored closely by a provider familiar with IL-2 therapy. Also, IL-2 therapy can be very expensive for the patient.”
Dr. Napolitano also discussed some of the contraindications and cautions that must be noted prior to IL-2 administration in HIV-positive patients. For example, patients with an active infection should delay IL-2 therapy, given that it can briefly suppress the immune response. Second, IL-2 administration should be avoided or approached with great caution in patients with cardiac or pulmonary disease, given the potential of IL-2 to cause hypotension and/or cardiopulmonary toxicity. “This includes patients with hypertension who are taking medications to control blood pressure,” Dr. Napolitano cautioned. “Medications used to control blood pressure may increase the risk of IL-2-associated hypotension.” Patients with autoimmune disease, diabetes, or thyroid disease may not be good candidates for IL-2 therapy, and need to be monitored very closely since IL-2 may worsen these conditions. HIV-infected women who are pregnant should not receive IL-2.
In summary, substantial increases in CD4+ cell counts associated with IL-2 therapy may, in theory, offer benefits to HIV-infected patients, such as decreased opportunistic infections, decreased bacterial and mycobacterial infections, and possibly enhanced HIV-specific immunity. “However,” Dr. Napolitano added, “there are insufficient data to determine if IL-2 confers true immunologic or clinical benefits. Moreover, with potential immunologic gain comes the cost of moderate toxicity, if the high-dose intermittent regimen is used.”
| Growth Hormone (GH) | Top of page |
While IL-2 appears to contribute to an increase in naive CD4+ cell populations through survival of existing cells in the periphery, research indicates that GH may increase the number of new T-cells much earlier in the production process. “We’re conducting studies in our laboratory to better understand how GH acts as an immune booster in the human body,” Dr. Napolitano explained. “Limited data suggest that it works very early in T-cell production by stimulating T-cell progenitors in bone marrow. It also may facilitate the engraftment and survival of these progenitors in the thymus.”
A number of rodent studies have been completed. GH may act directly upon immune tissues or its effects may be mediated indirectly through insulin-like growth factor-1 (igf-1). Rodents deficient in GH exhibit stress-related thymic hypoplasia that improves with GH replacement (Berczi, 1991; Murphy, 1992; Dorshkind, 2000). In older rodents, the administration of GH or igf-1 reverses age-related declines in thymopoiesis and accelerates immune reconstitution in immunodeficient animals (Kelley, 1986; Knyszynski, 1992; Bar-Dayan, 1994; Montecino-Rodriguez, 1998). What’s more, GH or igf-1 therapy accelerates immune reconstitution in immunodeficient animals (Beschorner, 1991; Murphy, 1992; Woo, 1999; Montecino-Rodriguez, 1998; Tian, 1998).
Based on the results of these rodent studies, Dr. Napolitano and her colleagues hypothesized that treatment with recombinant human growth hormone (rhGH) might reverse thymic involution and stimulate production of naive CD4+ cells in HIV-infected patients.
In a clinical trial published in a 2002 issue of AIDS, Dr. Napolitano’s team treated five HIV-infected adults with rhGH (Serostim) 3 mg/day for six to 12 months (Napolitano, 2002; 2002a). The dose was reduced to 1.5 mg per day after the first six months of therapy, except in one subject, in whom rhGH dose reduction occurred at month 2 as a result of persistent arthralgias. Immunological analyses were performed before rhGH treatment and repeated at three-month intervals after rhGH initiation. Thymic mass was analyzed using computed tomography with quantitative density and volume analysis. Analysis of circulating lymphocytes, including naive and memory T-cell subsets, was also performed using multiparameter flow cytometry.
There was a marked increase in thymic tissue in all subjects after six months of rhGH therapy (see Figure 3). At baseline, the five patients had thymic atrophy as evidenced by the near-complete replacement of the thymus by fat on ct scan (mean thymic index of one). Repeat analysis after six months of treatment revealed a prominent increase in dense thymus tissue in all of the rhGH recipients (mean thymic index of four, P = 0.0002 compared with baseline). Quantitative density and volume measurements at six months demonstrated a mean increase in thymic density of 86 Hounsfield Units (HU) (P = 0.0008), and a mean increase in thymic volume of 88% (P = 0.06). To determine whether the reversal of thymic atrophy was caused by a generalized lipolytic effect of rhGH, quantitative density analysis was performed on the axillary adipose tissue of each patient. No increase in axillary adipose density was detected (mean change of +3 HU at six months and -9 HU at one year).
Figure 3. Growth Hormone Therapy Is Associated with Increased Thymic Density and Volume
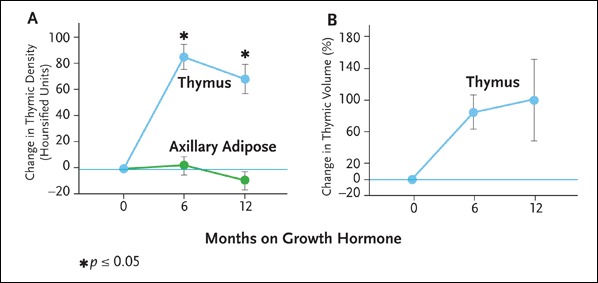
In a clinical trial reported in 2002, Dr. Laura Napolitano and her colleagues treated five HIV-infected adults with recombinant human growth hormone (rhGH) 3 mg/day for six to 12 months. The dose was reduced to 1.5 mg per day after the first six months of therapy, except in one subject, in whom rhGH dose reduction occurred at month 2 as a result of persistent arthralgias. Thymic mass was analyzed using computed tomography with quantitative density and volume analysis. Analysis of circulating lymphocytes, including naive and memory T-cell subsets, was also performed using multiparameter flow cytometry.
As is illustrated here, there was a marked increase in thymic tissue in all subjects after six months of rrhGH therapy. Quantitative density and volume measurements at six months demonstrated a mean increase in thymic density of 86 Hounsfield Units (HU), and a mean increase in thymic volume of 88%.
Source: Napolitano, 2002.
Treatment with rhGH was also associated with an increase in the percentage and absolute number of naive CD4+ cells. When compared with baseline values, the mean absolute gain in the naive CD4+ cell percentage was 6% at six months, 10% at nine months, and 12% at 12 months; the six, nine, and 12-month increases were statistically significant. Naive CD8+ cells did not increase with rhGH treatment, and no significant changes in thymic index, naive CD4+ cell percentages or naive CD8+ cell percentages were seen in historical control subjects who were maintained on effective antiretroviral therapy for a similar period of time.
Dr. Napolitano’s team followed two study participants to evaluate the effects of discontinuing rhGH on thymic function. Repeat thymus ct scans, three to twelve months after rhGH discontinuation, revealed a decrease to baseline thymic density in both individuals. However, gains in naive CD4+ cells remained stable.
A handful of studies reported over the past two years have supported the findings of Dr. Napolitano’s group. “It goes without saying that these findings do not support the general use of rhGH with the intent of reversing immune deficiency,” Dr. Napolitano said. Certain limitations of this study, including the small number of treated subjects and the lack of a randomized control arm, require that these data be interpreted with caution. There are also several significant adverse effects of growth hormone therapy that must be taken into consideration. Fortunately, additional studies are under way to evaluate further the role of rhGH as an immune-based therapy.
| Intereukin-7 (IL-7) | Top of page |
Interleukin-7 may also play a major role in sparking thymopoiesis and, as a result, an increase in naive CD4+ cells. In early rodent studies, very few T-cells were found in the thymus and peripheral lymphoid organs of mice lacking the IL-7 receptor (Peschon, 1994). Rodent studies have also found that IL-7 promotes more rapid immune reconstitution in mice after myeloablation or T-cell depletion (Morrissey, 1991; Bolotin, 1996; Mackall, 2001; Fry, 2001).
IL-7 appears to have several effects on T-cell production, judging by its effects on lymphoid progenitors in the bone marrow, thymocytes, and peripheral T-cells (see Figure 4). In the thymus, it inhibits apoptosis and enhances proliferation of developing thymocytes in the earliest stage of maturation. And in the periphery, IL-7 stimulates proliferation and enhances cytotoxicity of mature T-cells.
Figure 4. Interleukin-7: Effects on T-cell Production
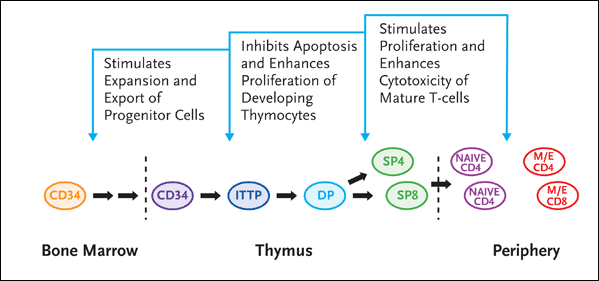
IL-7 appears to have several effects on T-cell production, judging by its effects on lymphoid progenitors in the bone marrow, thymocytes, and peripheral T-cells. In the thymus, it inhibits apoptosis and enhances proliferation of developing thymocytes in the earliest stage of maturation. And in the periphery, IL-7 stimulates proliferation and enhances cytotoxicity of mature T-cells.
Source: Laura Napolitano, MD.
To evaluate the activity of endogenous IL-7 in HIV-infection, Dr. Napolitano and her colleagues first set out to determine if HIV-mediated CD4+ cell loss induces the production of factors that are capable of stimulating lymphocyte development and expansion. In one series of cross-sectional (n = 168) and longitudinal (n = 11) analyses, her group demonstrated that increased circulating levels of IL-7 were strongly associated with CD4+ lymphopenia in HIV disease (Napolitano, 2001). Using immunohistochemistry with quantitative image analysis, Dr. Napolitano—working with Dr. Jan Andersson of the Karolinska Institute in Stockholm—also demonstrated that IL-7 is produced by dendritic-like cells within peripheral lymphoid tissues and that IL-7 production by these cells was significantly increased within lymphocyte-depleted tissues. This was characterized by an increased percentage of IL-7-positive cells and increased amounts of IL-7 produced per cell in biopsied lymph nodes.
Also of interest are unpublished data presented at a 2001 Keystone Symposium by Dr. Napolitano and Dr. Ruth Greenblatt of the Women’s Interagency HIV Study (WIHS). With pre- and post-antiretroviral therapy samples available from 237 HIV-positive women, Dr. Napolitano’s group confirmed its earlier results by demonstrating a strong and independent correlation between circulating IL-7 levels and lymphopenia. Of particular interest, pre-therapy IL-7 levels predicted the degree of CD4+ cell recovery upon initiating HAART, and post-therapy IL-7 levels decreased as the CD4+ cells recovered. These data indicate that endogenous IL-7 may function to increase lymphocyte production and/or expansion.
In more recent years, the results of non-human primate studies involving therapeutic administration of IL-7 have been reported. These studies have primarily involved administration of IL-7 to SIV-infected and uninfected macaques or to baboons undergoing bone-marrow transplants. In these animals, IL-7 was associated with a marked increase in circulating naive and memory CD4+ and CD8+ cells, splenomegaly, and lymphadenopathy. Given that there was no clear evidence of increased thymopoiesis, the predominant effect of IL-7 in these animals might be the expansion of peripheral T-cells.
Although IL-7 has been shown to enhance HIV replication in several laboratory studies, there was no acceleration of SIV infection in IL-7 treated animals, both on and off antiretroviral treatment. “Further animal studies are ongoing,” Dr. Napolitano commented. “Currently there are no data on the safety or immune effects of IL-7 in humans; however, clinical trials involving cancer patients are recently under way at the National Institutes of Health and a Phase I study is planned by the AIDS Clinical Trials group evaluating the tolerability of IL-7 in HIV-infected patients.”
| Conclusion | Top of page |
In summarizing her lecture, Dr. Napolitano reiterated that IL-2, GH, and IL-7 all appear to increase T-cell numbers, but through different mechanisms. “IL-2 mostly increases mature CD4+ cell populations in the periphery, whereas growth hormone appears to increase T-cell production primarily through effects on the bone marrow and the thymus. IL-7 may act at all three stages: in the bone marrow, thymus, and periphery. Understanding the mechanisms involved for each of these agents may become an important consideration when it comes to devising strategies to promote immune reconstitution in HIV-infected patients.”
Dr. Napolitano explained that the normal T-cell repertoire consists of approximately 100 million unique T-cell receptors. In the face of ongoing HIV replication, the diversity of the T-cell repertoire is gradually chipped away. Is it sufficient to employ therapies that can expand existing T-cells in the periphery to increase the number of mature cells with existing antigen specificities? Or is it more productive to spark bone marrow and thymic production of new CD4+ cells that can fill holes in the T-cell repertoire that are lost during HIV infection? Currently, there are no answers to these questions. “Future studies should help to address these issues,” Dr. Napolitano concluded.
| References | Top of page |
